-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
p-ISSN: 2331-7450 e-ISSN: 2331-7469
2018; 6(3): 60-64
doi:10.5923/j.jlce.20180603.02

Hirarchical Multi-ferroic FeBiO3 Nanoparticles Prepared by a Facile Solvothermal Method: Undergraduate Experiments for Inorganic Chemistry
Baoxiang Wang, Zhaobo Wang, Zhiyan Guo, Ruotao Feng, Fanglin Du
College of Materials Science & Engineering, Qingdao University of Science & Technology, Qingdao, China
Correspondence to: Baoxiang Wang, College of Materials Science & Engineering, Qingdao University of Science & Technology, Qingdao, China.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Wetting chemical method is a common method for the preparation of nano-materials, which are useful for experimental study by undergraduate students. Hierarchical multi-ferroic FeBiO3 nanoparticles were prepared by a facile solvo-thermal method and adopted as dispersing materials for electrorheological (ER) fluids. The morphology of FeBiO3 nanoparticles was characterized by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The structure was characterized by X-ray powder diffraction. Moreover, the electrorheological (ER) behavior of FeBiO3 nanoparticles was characterized using a rotational rheometer.
Keywords: Multi-ferroic material, Bismuth ferrite, Electrorheological fluid, Solvothermal method, Hirarchical Structure
Cite this paper: Baoxiang Wang, Zhaobo Wang, Zhiyan Guo, Ruotao Feng, Fanglin Du, Hirarchical Multi-ferroic FeBiO3 Nanoparticles Prepared by a Facile Solvothermal Method: Undergraduate Experiments for Inorganic Chemistry, Journal of Laboratory Chemical Education, Vol. 6 No. 3, 2018, pp. 60-64. doi: 10.5923/j.jlce.20180603.02.
Article Outline
1. Introduction
- Electrorheological (ER) fluids, acting as a type of smart material, exhibit a response to an applied external electric field where there occurs a rapidly reversible liquid to solid transition. An ER fluid is a two-phase suspension system typically composed of polarized particles dispersed in an insulating medium [1-3]. The ER effect, instantaneous and reversible changing in rheological properties of the fluids under the applied electric field, arises from the formation of chain-like or column-like structures between neighboring polarized particles along the direction of the applied electric field and results in a rapid transformation from a fluid-like state to a solid-like state [4-7]. Its response with and without an external electric field is shown as a schematic diagram as Figure 1. The application of multiferroic materials in electrorheological fluids is important but is seldom.
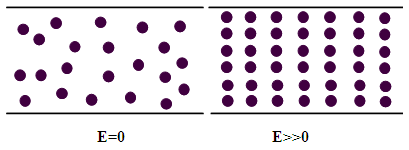 | Figure 1. Schematic illustration of the particles change of an ER fluid before and after an external electric field is applied |
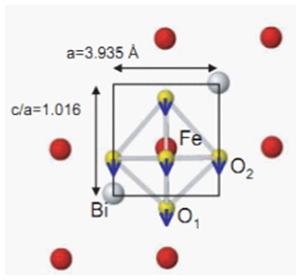 | Figure 2. The structure of Bismuth ferrite |
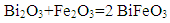 | (1) |
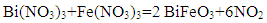 | (2) |
2. Experiment Description
2.1. Experimental Materials
- Fe(NO3)3 (Beijing Chemicals, Beijing, China), Bi(NO3)3 (Sinopharm Chemical Reagent Corporation, Shanghai, Chian), cetyltrimethyl ammonium bromide (CTAB, Tianjin Bodi Chemical Co. Ltd., Tianjin, China), Ethanol glycol (EG, Laiyang Fine Chemical Factory, China), anhydrous methanol (Tianjin Fuyu Fine Chemical Co. Ltd., Tianjin, China) and silicone oil (Tian Jin Damao limited company, Tianjin, China) were purchased. All reagents were of analytical grade and used as received without further treatment.
2.2. Instruments and Characterization
- The morphology of obtained BFO nanoparticles were observed by field emission scanning electron microscopy (FE-SEM, JEOL-670) and transmission electron microscopy (TEM, JEOL-2100). X-ray diffraction (XRD, Rigaku D/MAX-2500/PC) was performed to analyze the crystalline structure of as-prepared samples. The rheological properties of ER fluids were measured using a rotational rheometer (HAAKE Rheo Stress 6000, Thermo Scientific, Germany) equipped with a parallel-plate system (PPER35, 35 mm in plate diameter and 1 mm in gap width) and a WYZ-020 DC high-voltage generator (voltage: 0–5 kV, current: 0–1 mA) under a controlled shear rate (CSR) mode ranging from 1 to 500 s-1.
2.3. Experimental Procedure
- The hierarchical multi-ferroic FeBiO3 nanoparticles were prepared by using a facial solvothermal route [12]. In a typical procedure, 20.2g Fe(NO3)3 and 24.25g Bi(NO3)3 were added into a 120mL EG solution together under vigorously stirring to obtain a homogeneous solution. Then 5g CTAB was added into 30mL EG, followed by stirring for another 30 min. Then the two prepared EG solutions were mixed together and stirred for about 1h. Subsequently, the clear solution was transferred into a 100mL Teflon-lined stainless-steel autoclave, which was sealed tightly and heated at 200°C for 12 h. After cooling down to room temperature naturally, the resulting white precipitate was harvested by centrifuging and washed with ethanol for 3 times, the FeBiO3 products were collected and dried at 80°C overnight.
3. Results and Discussion
- The crystalline structures of FeBiO3 nanoparticles were analyzed using an X-ray diffractometer. As shown in Fig. 3, the pattern of Ti-containing precursor is to be readily indexed to perovskite (space group R3c) BiFeO3 (JCPDS Card No. 20-0169) and no peaks from other phase were detected, demonstrating that well-crystallized single phase BiFeO3 can be obtained under the current synthesis conditions. These reflections correspond to crystal planes 2θ=22.490(101), 31.808(012), 39.509(021), 45.813(202), 51.376(113), 57.012(122), 67.087(220), 71.338(303), 76.082(312) (PDF#20-0169).
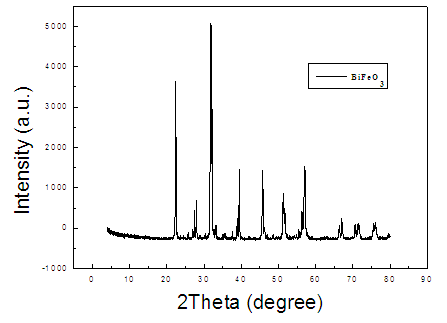 | Figure 3. XRD pattern of as-synthesized FeBiO3 sample |
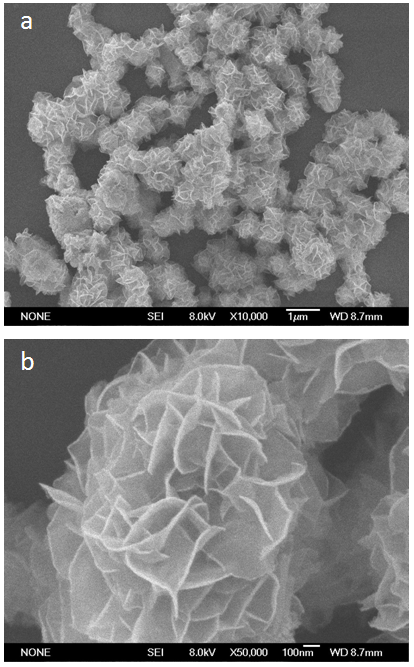 | Figure 4. SEM images of as-prepared FeBiO3 |
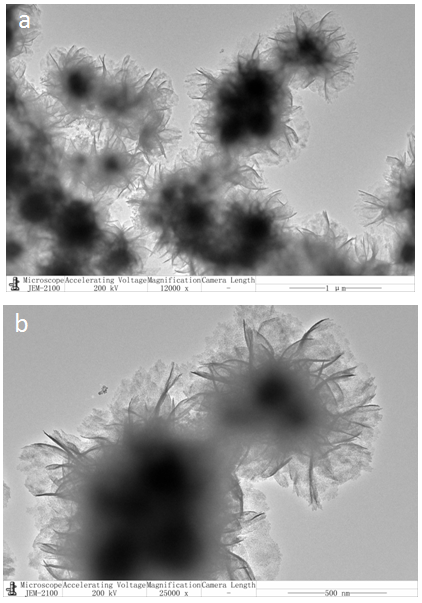 | Figure 5. TEM images of FeBiO3 nanoparticles |
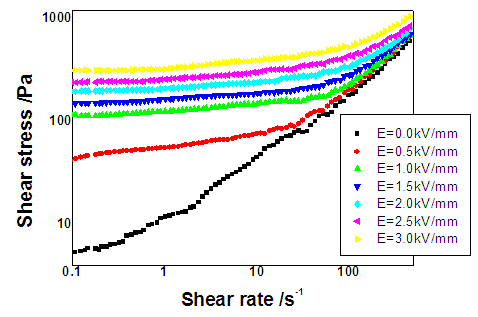 | Figure 6. Flow curves of shear stress as a function of shear rate for FeBiO3 nanoparticles the suspensions |
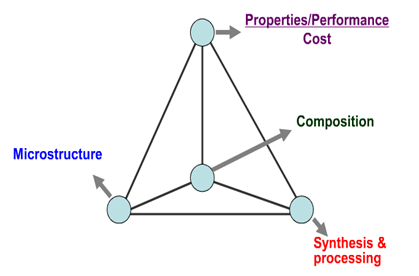 | Figure 7. Schematic diagram of Material Four Element Tetrahedron |
ACKNOWLEDGEMENTS
- This work was financially supported by Upgraded Project of Shandong Province for Guidance Ability of Graduate Tutors (SDYY17047; SDYY17044; SDYY16014), and Training Programs of Innovation and Entrepreneurship for Undergraduates, China (201710426089).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML