-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
p-ISSN: 2331-7450 e-ISSN: 2331-7469
2018; 6(2): 36-45
doi:10.5923/j.jlce.20180602.04

Making the Learning of Acid-Base Concepts More Relevant - A Research Study
Muhamad Hugerat1, Naim Najami1, Riam Abu-Much1, Wedad Khatib1, Avi Hofstein2
1The Academic Arab College for Education in Israel – Haifa, Haifa, Israel
2Department of Science Teaching, The Weizmann Institute, Rehovot, Israel
Correspondence to: Muhamad Hugerat, The Academic Arab College for Education in Israel – Haifa, Haifa, Israel.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This study examines how learning acid-base concepts can be made more relevant for students. It analyzes how the concepts learned in chemistry by tenth graders are aligned with their everyday lives. Two approaches to teaching are compared: the Low Relevance Approach (LRA) and the High Relevance Approach (HRA). The HRA approach emphasizes learning how chemistry materials are relevant to students’ daily life.The subject taught was acids and bases. Following the intervention program, a perception-type motivation and satisfaction questionnaire was administered to the students in order to determine the effect that the ‘relevant experiential’ teaching style had on students’ attitudes towards chemistry, regarding their motivation for and satisfaction when studying chemistry, and on their academic achievements in the chemistry. Based on the assessment of students' cognitive and scholastic performance, it is clear that relevance-oriented experiments in chemistry significantly contribute to these learning variables.
Keywords: Introductory, Laboratory Instruction, Hands-On Learning, Pedagogy, Acids / Bases Topics
Cite this paper: Muhamad Hugerat, Naim Najami, Riam Abu-Much, Wedad Khatib, Avi Hofstein, Making the Learning of Acid-Base Concepts More Relevant - A Research Study, Journal of Laboratory Chemical Education, Vol. 6 No. 2, 2018, pp. 36-45. doi: 10.5923/j.jlce.20180602.04.
Article Outline
1. Introduction
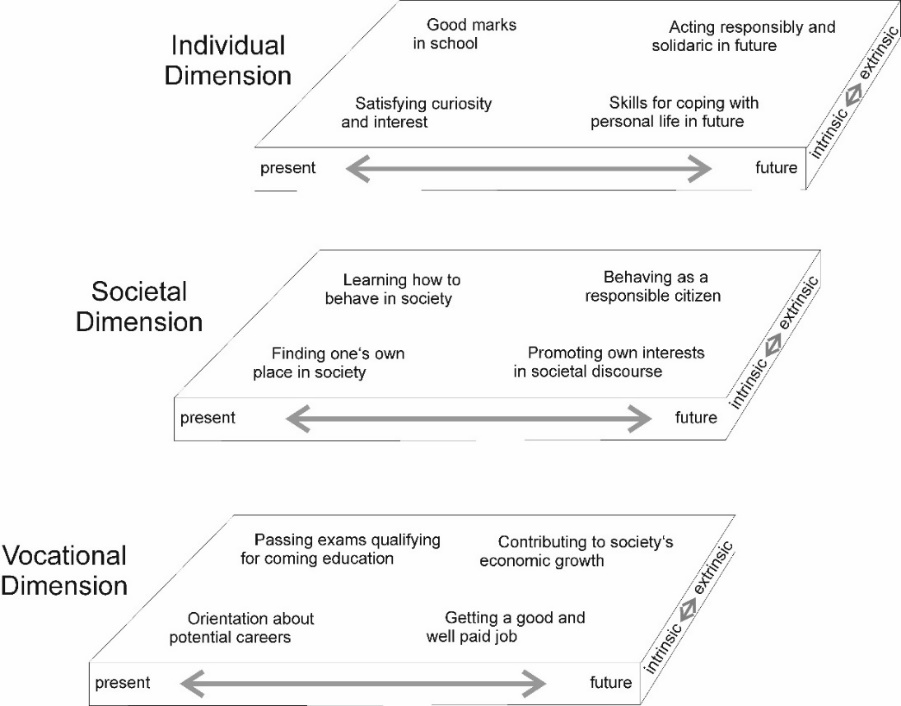
- Bratz [1] wrote that no evidence exists to support the hypothesis that instruction designed in response to students’ learning styles can improve achievement. On the other hand, Towns [2] described how Kolb's learning styles were particularly well suited to learning in the chemistry laboratory. Pashler and colleagues [3] reported that learning is optimized when the instruction matches the learner's preferences and abilities.Many studies have presented a gloomy picture with respect to the learning styles of science, especially at the secondary school level. A key claim is that science education - particularly in physics and chemistry - remains unpopular among students. Several of these studies [4-6] infer that students are insufficiently interested in chemistry and/or not motivated to learn chemistry concepts and topics. Learners were frequently found to perceive chemistry as “irrelevant” both for themselves and for the society in which they live. [5, 7, 8] Science teachers in general, and chemistry teachers in particular, are urged to make learning science (chemistry in our case) “more relevant” in order to better motivate their students and interest them in learning chemistry. [9-12] However, it may be unclear to instructors what exactly is meant by “making science learning more relevant”. The connections (or differences) among terms such as relevance, interest, and motivation may need clarification. Recently, Stuckey et al. [13] published a review and Eilks and Hofstein edited a book on the issue of relevance regarding the teaching and learning of chemistry in which the concept of relevance is analyzed and a schematic framework for relevance in science learning is suggested [14, 15]. Three dimensions for relevance in science education are proposed: individual, societal, and vocational [13]. Here is a short version of these characteristics: • The individual dimension: This focuses on matching the subject matter to the learners’ curiosity and interests, providing students with necessary and useful skills for coping with their everyday lives today and in the future, and contributing to pupils’ development of intellectual skills. • The societal dimension: This focuses on preparing pupils for self-determination and on achieving a responsibly led life in society by understanding the interdependence and interaction of science and society, and by developing skills for societal participation and competencies in order to contribute to society’s sustainable development.• The vocational dimension: This focuses on offering orientation for future professions and careers, preparing for further academic or vocational training, and opening up formal career chances (e.g., by having sufficient coursework and achievements to enter into institutions of higher education).These dimensions are not fully complementary or dichotomized. They are interrelated and partially overlap. For example, either career orientation can match personal curiosity or it can answer a demand for more scientists and engineers in the future. The latter is directly linked to the idea of the prosperous and sustainable development of society. The model [14], which has been slightly modified, illustrating the different dimensions of the relevance of science education (Figure 1); it can easily be adapted and interpreted also for the domain of chemistry education, since it constitutes part of science education in general.
2. The Study
- As mentioned before, chemistry education in secondary school is often seen as divorced from real life. It usually takes place in a school classroom or laboratory setting using abstract concepts and unfamiliar language (the language of chemistry). As a result, students do not see the relevance, interest, and/or importance of their everyday life outside of school or their future role in society. [22] Thus, chemistry topics taught in schools should help students understand and use basic chemical concepts as well as relate these concepts to real world issues. In addition, the lessons should demonstrate how chemistry is used for understanding and controlling issues such as food chemistry, climate change (acid rain) energy consumption, and others. The current study’s main objective was to seek, understand, and propose methods that would enable chemistry teachers to acquire tools for teaching the sciences in general and chemistry in particular (specifically acids and bases) in a way that would enhance students’ curiosity and enable them to associate the study materials with their own personal lives and to the society in which they operate. In the following study we adapted two pedagogical approaches for teaching chemistry concepts (see Figure 2) the Low Relevance Approach (LRA) (Traditional Process) that is mainly based on a conceptual approach and often ends there, and the High Relevance Approach (HRA) (Alternative Process) that is mainly based on students' experiences in their lives, for example, materials used in kitchen chemistry, food chemistry, and others, These two approaches were statistically compared.
 | Figure 2. Two curriculum approaches in chemistry (Reprinted with permission from ref. 22, ©2015 Sense Publishers: Rotterdam) |
3. Methodology
- The Low Relevance Approach (LRA)One of the most key concept in teaching chemistry in school is the acid-base topic. [23] In accordance with this method, the student learns about acids and bases in a method sequence of lessons, activities, experiments, and cover content to meet course outcomes. Here the materials used in the experiments are not from daily life, such as HNO3, H2SO4, NaOH, and HCl. Students learn about titration between an acid (HCl) and a base (NaOH) when the indicator is Phenolphthalein or litmus paper, materials unfamiliar to them from their experience in everyday life. Additionally, students learn about the pH scale, different types of acids and bases, and the reactions between them; however, the students encounter none of these topics in their daily life. Students also learn about the preparation of acids and bases caused by the reaction between different chemical oxides and water; here too, none of these materials are known from their everyday life (more details about the lessons, activities and experiments you can find in the supporting information).The LRA is abstract teaching; it is traditional process [22], which contain sequence of lessons, experiments, cover content to meet course outcomes (Fig. 2, Fig. 3). This method does not support the individual, social and vocational dimension according to the model (Fig. 1) [14] described by Stucky et al. [13]The High Relevance Approach (HRA)In our everyday lives we use various materials for different purposes, which we usually take for granted and do not consider what life would be like without them. One interesting exercise is to get students to think what their day would be like without any products produced by chemistry. These students study the acid-base concept in a manner relevant to their everyday lives, using materials such as vinegar as an acid and baking soda solution as a base, materials that are used every day or that represent the chemistry of everyday objects. The indicator that is used to identify the acids and bases and the subject of titration is red cabbage juice, which is derived from red cabbage salad, a popular salad in Israel. It should be emphasized here that the student uses materials with which they are familiar, especially from the kitchen [24, 25]. Here in Israel, the most popular and well-known salad is red cabbage salad. There is hardly a meal in Israel that does not contain this salad, and on the other hand, it is one of the main ingredients in the falafel sandwich (actually pita), which is considered the Israeli national food. In addition, hardly any Israeli kitchen does not contain white vinegar, baking soda, and lemon juice, which are added to the red cabbage salad. The students also learn about acid production using as an example the acid rain that is very common in the Haifa Bay region, which has a large number of chemical plants. Sulfur dioxide - SO2 – is emitted by the refineries at Gadiv, Nadiv, Carmel Olefins, and Nesher. It is emitted because of the burning of fuel oil with high sulfur content. Until 2014, it was also emitted from the electricity company’s chimneys.Chemicals and fertilizers such as nitrogen oxides - NOx - are emitted from Haifa during the process of producing nitric acid (HNO3).Students build their own pH scale from the obvious visible changes using the red cabbage juice along with acids and bases from their daily life. Preparing an indicator requires using extracts of red cabbage. In this activity, the social side of the activity is strengthened when the students need to cut the red cabbage and then discover that their hands are stained. They must collaborate to decide how to prepare an indicator solution from it and they discover that the color varies depending on the acidic/basic solution. The relevant part of this method is that students collaborate and think together to create an indicator from red cabbage, which is familiar to every resident in Israel (more details about the lessons, activities and experiments you can find in the supporting information).The HRA is alternative process [22], which contain sequence of lessons, experiments using familiar materials and focus in the students' everyday experiences (Fig. 2, Fig. 3). This method supports the individual, social and vocational dimension according to the model (Fig. 1) [14] described by Stucky et al. [13]
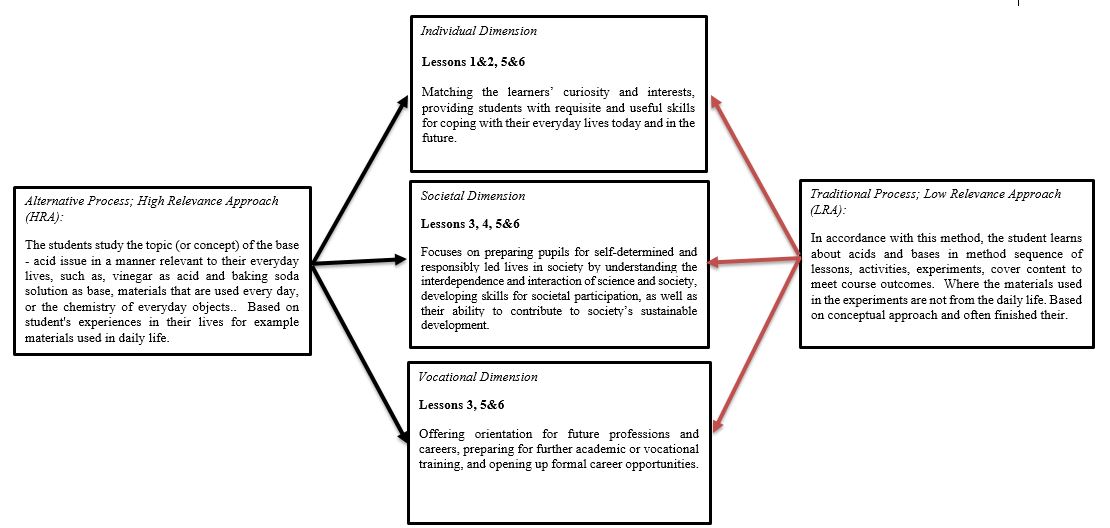 | Figure 3. Comparison between the two methods; HRA, which support, individual, social and vocational dimension; and LRA, which does not supports these dimensions |
4. Assessment Measures
- The research tool consists of a structured questionnaire with three parts.Part One: Structured questionnaire for measuring students’ level of motivation For use with the students in this study, a questionnaire of 30 statements was developed from Glynn & Koballa’s Science Motivation Questionnaire [26]. This questionnaire has a reliability (internal consistency) of α = 0.93 [26] and tests six different aspects of motivation with statements describing situations and behaviors of students. In order to adapt the questionnaire to be used in the Arabic schools, it was translated to Arabic and the content was validated by a group of faculty members in the Arabic College, both for the language as well as for the content.The variable of “motivation” was determined by calculating the mean of participants’ responses to the 30 items. Every student received a motivation score ranging between 1 and 5; the higher the score, the greater the motivation, and vice versa. The motivation questionnaire’s reliability was checked by means of an internal consistency test using Cronbach’s alpha reliability coefficient. The result, α = 0.89, indicates high reliability.Part Two: Structured questionnaire for measuring student attitudes towards the sciences The questionnaire was adapted from (translated to Arabic from Hebrew) Abadi & Kashtan [27] and consists of 22 statements that measure students' attitudes towards the sciences that include depicting situations, personal feelings, and beliefs. All students were asked to report the extent to which they agree with each of the beliefs and concepts in the questionnaire, using a four-item Likert scale: 1 – do not agree at all (oppose); 2 – disagree; 3 – agree; 4 – very much agree. The reliability coefficient was α = 0.83.Part Three: Open questions related to students’ perceptions regarding "relevance"In addition to the above-described quantitative measures, the students also underwent a structured interview questionnaire in order to back up the quantitative findings and to shed more light on the research topic. This questionnaire consists of six open-end questions in which the students are requested to describe their attitudes, opinions, and feelings towards their participation in the intervention program and towards instruction of science by means of the relevance approach. The answers were analyzed using content analysis and cross-checked with the quantitative findings. Several members of the college staff validated the content to ensure the accuracy and validity of its translation into Arabic (from English and Hebrew).
5. Research Design and Procedure
- This research is a ‘pre-post intervention’ and an ‘experimental-control’-oriented study. Research SampleThe study population consists of two groups, each composed of tenth-grade classes. The two groups were nearly identical regarding the features relevant for the study. The questionnaires were administered to the two groups at the beginning of the study (intervention). Then one group was defined as the control group, which would be taught using the LRA, and the other was defined as the experimental group, which would be taught using the HRA in an intervention program. In order to test the independent variable and determine whether it underwent any change, the attitude and motivation questionnaires were once again given to both groups and data on them were collected by means of the concluding questionnaire. A sample group of students was then selected to fill in an open-ended questionnaire.The data were analyzed in accordance with the principles of quantitative research. In order to test for variance within and between the groups regarding motivation, an F-test for variance was carried out before and after the experiment; in order to test for variance within and between the groups regarding the variable of satisfaction before and after the experiment, a t-test was conducted on two independent samples, to determine whether the variance between the (experimental and control) groups was significant. In addition, a Pearson correlation coefficient was used to determine whether and how the variable of achievement correlated with the variables of attitudes and motivation.
6. Results
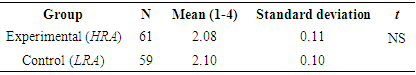
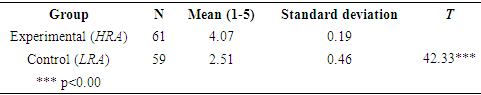
- The hypothesis that teaching topics in chemistry (acids and bases) by using the relevance method (HRA) brings about an improvement in students’ attitudes in comparison with students who were instructed using the LRA was tested in three stages. The first stage took place before the intervention program was implemented; it involved testing for differences in attitude towards chemistry and its study among the students in the experimental (HRA) and the control groups (LRA), in order to determine both groups' initial stage before one of them participated in the intervention program. The difference between the two groups was determined by means of a t-test for two independent samples.
|
|
|
|
|
|
|
|
7. Discussion
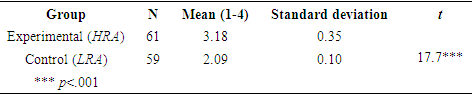
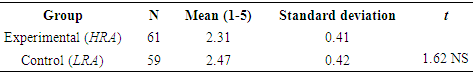
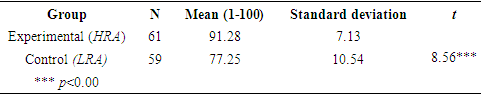
- Some students who study chemistry do so because they wish to devote their future to the mathematical analysis of chemical processes. Others are enticed in studying the human body, among other reasons. Indeed, interest in the subject matter plays a crucial role in enhancing motivation and success among learners. It is important therefore, to emphasize that teaching the topic of acids and bases by means of HRA can attain this goal.In this study, the researchers examined how studying chemistry (specifically, acids and bases) by means of the HRA correlates with improved student academic achievements in chemistry, attitudes towards science, motivation, and satisfaction. Relevance is an increasingly important factor because, based on the declaration made by the Ministry of Education in Israel, quite a few reforms and changes are being carried out in Israel in order to promote teaching that places the student in the center. The value of the present study lies in its highlighting the need to create relevance when teaching science to students; its findings show that emphasizing the learned subject's relevance can generate a process of improving a learners' achievements in knowledge-type tests.The study also demonstrates that an intervention program in which students in the experimental group (HRA) are taught the topic of acids and bases by means of the relevance method improved students’ attitudes towards the sciences and studying chemistry and supports the three dimensions: individual, social, and vocational. This post-intervention improvement in the results of the experimental (HRA) compared to the control group (LRA) is statistically significant (Tables 6 and 9). These findings are in agreement with those of other studies [28-30] in which students’ attitudes towards science and mathematics were tested, with an emphasis on students' attitudes towards and love of science, the significance that they ascribe to the subject, and their degree of self-confidence as learners.Students in the experimental group (HRA), in which the subject of acids and bases was instructed by means of the relevance method, experienced a rise in their level of motivation and satisfaction with respect to the study of science, in comparison with students who were instructed by means of LRA. The difference between the two groups was significant. This finding is in accordance with other studies [31] in which the highest quality of conceptual learning occurred under conditions of motivation that promotes personal growth and a reshaping of one's worldview.Above all, this study demonstrates a significant improvement in academic achievement in the field of chemistry among students who were instructed by means of the HRA in comparison with students who were instructed by means of the LRA (Table 7).The findings also highlight the importance of the HRA in teaching acid-base concepts, in line with studies on meaningful, relevant experiential learning, which emphasizes that the creation of meaning must begin immediately after the student enters school, by associating the subject matter with the child's experiences and home culture. Bruner affirms that culture affects learning by providing children with a toolbox with which they can build up their world and their personal conceptions and understanding. [32] Therefore, teachers must become acquainted with children's actual world and the influences that shape them. [33] In an example from the LRA in teaching acid-base concepts, the instructor announced that today's lesson would be about "titration of acid "HCl" and base "NaOH" using Phenolphthalein as an indicator for acids and bases". The students were then asked: "What is the connection between phenolphthalein and the indicator for acids and bases and daily life." This question initiated a context-based learning process. One of the student's reactions was: "I have no idea about the relation between HCl, NaOH and phenolphthalein; in the future if I will become a teacher I will not teach this topic, because my student will not connect this topic to daily life ". Another student's reaction was: "I don’t want to become chemistry teacher in the future; the subject has no connection to our daily life". These students’ reactions clearly indicate that using LRA in teaching the acid-base topic does not supports the vocational dimension. From other students’ reactions its clearly that using the LRA in teaching the acid-base topic does not supports the social, individual and vocational dimension.In an example from the HRA in teaching acid-base concepts, the instructor announced that today's lesson would be about "red cabbage juice", as one example of an indicator for acids and bases. The students were then asked: "What is the connection between red cabbage juice and the indicator for acids and bases." This question initiated a context-based learning process. One of the student's reactions was: "Only now do I understand how I will become a different teacher in the future". Another student's reaction was: "Now, I want to be researcher in the field of food chemistry". These students’ reactions clearly indicate that using HRA in teaching the acid-base topic supports the vocational dimension.In another example from the HRA in teaching the acid-base topic, the instructor announced that today's lesson would be about "acid rain", as one example of producing acids. The students were then asked: "What is the connection between acid rain and the production of acids?" This question initiated a context-based learning process. One of the student's reactions was: "Now I will be more active in the future in the field of environmental protection to save our planet". Another student's reaction was: "I want to be a researcher and develop methods that will reduce the amount of gases responsible for acid rain ". These students’ reactions clearly indicate that using the HRA in teaching the acid-base topic supports the social dimension and the vocational dimension.In another example from the HRA in teaching the acid-base topic, the instructor announced that today's lesson would be about "is every transparent liquid water?" as one example of acid-base. The students were then asked: "What is the connection between this topic and the acid base one." This question initiated a context-based learning process. One of the student's reactions was: "This topic increased my interest and curiosity to think about the subject of acids and bases". Another student's reactions was: "This topic made me think deeply of transparent and acidic fluids encountered in everyday life". These students’ reactions clearly indicate that using the HRA in teaching the acid-base topic supports the individual dimension.The Ministry of Education designs the curriculum. The officials responsible for its implementation try to link its objectives to the students' world. However, not every student comes to class with a burning desire to “reach the summit”, so part of the teacher's job is to make the information and skills accessible to the students from their standpoint. However, it is no easy task to make topics in chemistry or biology understandable to most students; this requires considerable thought and careful planning, and requires lesson plans that are flexible enough so that they can be adapted to the learning population and to developments in the classroom. For this reason, the researchers of this study wish to stress the need for relevance and interest in order to improve learning and enhance the learners' sense of belonging to the science curriculum and to the scientific community. Therefore, it is essential to continue to build bridges between the curriculum and the students' world in order to narrow the gap in learning.
8. Conclusions
- The present study's main conclusion is that teaching a topic using a relevancy-oriented method enhances the levels of student motivation and satisfaction and improves students’ attitudes towards science and its learning. For this reason, the authors highly recommend the use of the relevance method for teaching science as well as to include laboratory experiments in order to make the learning process more experiential and significant. This research focuses on the relevance of chemistry education. It was inspired by a recently suggested definition and model of the relevance of science education in its adjustment to the teaching and learning of chemistry. [13-15, 21, 22]This study is also in agreement with the recommendation made by Fensham, [27] who urges adopting science education methods based on socio-scientific issues, despite the method's social emphasis. Fensham contends that this type of science education helps students learn and understand the interconnections between science and society and helps them develop skills that will encourage them in the future to participate in scientific discussions and decision-making processes in society. In addition, with this type of science education students are also required to learn intellectually challenging scientific skills. For this reason, the proposed method of this research is imperative, since it also presents students with various possible paths for career development.The amount of instruction may be varied to suit different age groups. For this reason, Fensham developed a general structure for a twelve-year science curriculum, [33] with different emphases for different age groups. During the first five years, the emphasis is on "wonder and creativity". Subsequently the context moves towards science, civics, and emphasis on self-awareness and decision-making. The professional dimension is combined with the changing emphases. [33] However, since this study focuses on one specific topic (acid-base), it is advisable to test the effects of the intervention program on other, more complex topics over a longer period. In light of these findings, it can be concluded that teaching chemistry using the HRA method increased student motivation and satisfaction and improved their attitudes towards chemistry and its study, and supported the three dimension: individual, social, and vocational. On the other hand, the intervention program in which chemistry was taught to the experimental group (HRA) using the relevance method improved students' achievements when studying chemistry.
9. Associated Content
- Supporting InformationThis part contain the lessons, activities and experiment, which will be implemented during the Low Relevance Approach (LRA) and the High relevance Approach.
References
| [1] | Bretz, S. L. Finding No Evidence for Learning Styles. J. Chem. Educ. 2017, 94, 825-826. |
| [2] | Towns, M. H. Kolb for Chemists: David A. Kolb and Experiential Learning Theory. J. Chem. Educ. 2001, 78 (8), 1107. |
| [3] | Pashler, H.; McDaniel, M.; Rohrer, D.; Bjork, R. Learning Styles: Concepts and Evidence. Psychol. Sci. Public Interest 2008, 9 (3), 105-119. |
| [4] | Hofstein, A., Eilks, I.; Bybee, R. Societal issues and their importance for contemporary science education: A pedagogical justification and the state of the art in Israel, Germany and the USA. International Journal of Science and Mathematics Education 2011, 9, 1459-1483. |
| [5] | Holbrook, J.; Rannikmae, M.; Kask, K. Teaching the PARSEL Way: Students' Reactions to Selected PARSEL Modules. Science Education International 2008, 19 (3), 303-312. |
| [6] | Osborne, J., & Dillon, J. Science Education in Europe: Critical Reflections 2008. London: The Nuffield Foundation. |
| [7] | Dillon, J. On Scientific Literacy and Curriculum Reform. International Journal of Environmental & Science Education 2009, 4, 201-213. |
| [8] | Gilbert, J. K. On the Nature of “Context” in Chemical Education. International Journal of Science Education 2006, 28, 957-976. |
| [9] | J Holbrook, J. Increasing Relevance of Science Education: The Way Forward Science Education International 2003, 14 (1), 5-13. |
| [10] | Holbrook, J. Making Chemistry Teaching Relevant. Chemical Education International 2005, 6(1), 1-12. Retrieved from http://old.iupac.org/publications/cei/vol6/06_Holbrook.pdf. |
| [11] | Newton, D. P. Relevance and science education. Educational Philosophy and Theory 1988a, 20 (2), 7-12. |
| [12] | Newton, D. P. Making Science Education Relevant 1988b. London: Kogan Page. |
| [13] | Stuckey, M.; Mamlok-Naaman, R.; Hofstein, A.; Eilks, I. The Meaning of ‘Relevance’ in Science Education and its Implications for the Science Curriculum. Studies in Science Education 2013, 49, 1-34. |
| [14] | Eilks, I.; Hofstein, A. From some historical reflections on the issue of relevance of chemistry education towards a model and an advance organizer – A prologue. In Relevant Chemistry Education – From Theory to Practice; Eilks, I., Hofstein, A.; ©2015 Sense Publishers: Rotterdam, 2015, pp 1-10. |
| [15] | Hugerat, M.; Mamlok-Naaman, R.; Eilks, I.; Hofstein, A. Professional development of chemistry teachers for relevant chemistry education. In Relevant Chemistry Education – From Theory to Practice; Eilks, I., Hofstein, A.; ©2015 Sense Publishers: Rotterdam, 2015, pp 369–386. |
| [16] | Burmeister, M.; Rauch. F.; Eilks, I. Education for Sustainable Development (ESD) and Secondary Chemistry Education. Chemistry Education Research and Practice 2012, 13, 59-68. |
| [17] | Roth, W.-M.; Lee S. (2004). Science Education as/for Participation in the Community. Science Education 2004, 88, 263-291. |
| [18] | Nentwing, M. P., Demuth, R., Parchmann, I., Ralle, B., Grasel, C. Chemie im Kontext: Situating learning in relevant contexts while systematically developing basic chemical concepts. Journal of Chemical Education 2007, 84(9), 1439. |
| [19] | Kirk, R. S., Silverstein, P. T., Willemsen, J. J. Teaching Biological relevant chemistry throughout the four-year chemistry curriculum. Journal of Chemical Education 2006, 83(8), 1171. |
| [20] | Stacy, M. A. Biologically relevant chemistry in the freshman laboratory. Journal of Chemical Education 1995, 72(6), 533. |
| [21] | Garforth, F. Chemistry through the looking glass. In Everyday chemistry; P. E. Childs, P. E. (ed.); Limerick, Thomond College, 1986, pp.4-45. |
| [22] | Childs, P. E.; Hayes, S. M.; O’Dwyer, A. Chemistry and everyday life: Relating secondary school chemistry to the current and future lives of students. In Relevant Chemistry Education – From Theory to Practice; Eilks, I., Hofstein, A.; ©2015 Sense Publishers: Rotterdam, 2015, pp 33-54. |
| [23] | Hoving Kouyoumdjian, C.; Uunderwood, S.M. Investigating Students' about Acid-base Reactions Melanie. J. Chem. Educ. 2016, 93(10), pp 1703-1712. |
| [24] | Hugerat, M.; Basheer, S. Is Every Transparent Liquid Water?. Journal of Chemical Education 2001, 78, 1041. |
| [25] | Hugerat, M. The Magic Liquid- a Science Story about Acids and Bases. The Science education Review 2006, 5(4), 111-114. |
| [26] | Glynn, S.M.; Koballa, T.R. (2006). Motivation to Learn in College Science. In: Mintzes, J.J. & Leonard, W.H. (Eds.). Handbook of college science teaching: theory, research, practice. Arlington: NSTA Press. 2006, pp. 25-32. |
| [27] | Abadi, R.; Kashtan, Y. Factors of Success in Junior High school Science Studies, Research report, MOFET institute. 2001, Retrieved from: http://www.mofet.macam.ac.il/infocenter/Pages/ResearchTools/635096616679811284.aspx (In Hebrew). |
| [28] | Nahmias, R.; Zozovsky, R. Academic achievement and educational context of eighth-grade students in Israel in mathematics and science. 2002, TIMSS 2007. Jerusalem and Tel-Aviv: Tel-Aviv University and RAMA. (In Hebrew). |
| [29] | Martin M. O.; Mullis, I. V. S.; Foy, P.; Stanco, G. M. TIMSS 2011 International Results in Science. Chestnut Hill, MA and Amsterdam: TIMSS & PIRLS International Study Center and IEA. 2012. |
| [30] | Mullis I. V. S.; Martin M. O.; Foy, P.; Arora A. TIMSS 2011 International Results in Mathematics. Chestnut Hill, MA and Amsterdam: TIMSS & PIRLS International Study Center and IEA. 2012. |
| [31] | Lepper, M. R., Corpus, J. H., & Iyengar. S. S. Intrinsic and Extrinsic Motivational Orientations in the Classroom: Age Differences and Academic Correlates. Journal of Educational Psychology 2005, 97, 184-196. |
| [32] | Bruner, J. S. The culture of education. Cambridge, MA: Harvard University Press. 1996. |
| [33] | Fensham, P. Science for all: What Have we been Teaching in the Science Curriculum and What Should we Teach?. New Horizons in Education 2004, 111, p. 28 – 43. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML