-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
p-ISSN: 2331-7450 e-ISSN: 2331-7469
2018; 6(2): 31-35
doi:10.5923/j.jlce.20180602.03

Using Mercury and CrystalDiffract to Introduce Crystallography Concepts at the Undergraduate Level
Peter J. Rosado Flores
Department of Chemistry, Physics, and Astronomy, Georgia College and State University, Milledgeville, GA, United States
Correspondence to: Peter J. Rosado Flores, Department of Chemistry, Physics, and Astronomy, Georgia College and State University, Milledgeville, GA, United States.
| Email: |  |
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Chemical crystallography is considered one of the essential characterization techniques of inorganic chemistry since it is a critical tool in the structural determination of molecules small and large. Several attempts have been made to introduce crystallography topics at the undergraduate level, but the one present obstacle is the availability of instrumentation for undergraduates to run experiments in. State of the art X-ray diffraction instruments typically reach the ~$500,000 range and are rarely available to primarily undergraduate institutions. While accessibility to high-tech instrumentation is indeed an obstacle, there are educational sources that are quite accessible for undergraduate students, merely requiring a working computer to make use of them. Thus, the aim of this article is to touch upon the attempts to introduce the topic of crystallography to undergraduates of the inorganic chemistry laboratory at Georgia College and State University (GCSU). Two guided inquiry research type assignments are herein discussed that expose students to crystallography and enhance literature review/analysis skills that allows them to become better undergraduate researchers.
Keywords: X-ray crystallography, Structure determination, Theoretical crystallography, Undergraduate research
Cite this paper: Peter J. Rosado Flores, Using Mercury and CrystalDiffract to Introduce Crystallography Concepts at the Undergraduate Level, Journal of Laboratory Chemical Education, Vol. 6 No. 2, 2018, pp. 31-35. doi: 10.5923/j.jlce.20180602.03.
Article Outline
1. Introduction
- Ever since the discovery of X-rays by Rӧntgen, [1] crystallography has become an important tried and true way for structural determination. According to the Cambridge Crystallographic Data Centre (CCDC) [2], over 900,000 examples of approved and revised crystal structures have been entered since its inception. Therefore, it is a true fact that crystallography be included in the undergraduate chemistry curriculum. Single Crystal X-Ray Diffraction (SCXRD) and Powder X-Ray Diffraction (PXRD) may seem like topics out of the grasp of undergrads, but there exist a variety of software and resources accessible to primarily undergraduate schools (PUIs), without breaking departmental budgets. In the past, several attempts have been made to introduce crystallography at the undergraduate level in classrooms and laboratories alike. [3-6] Yet some of these require pre-existing data sets that might be difficult for instructors to acquire who have not had previous exposure (or performed some structure determination in the past). In addition, instrumental costs can reach the $500,000 mark. This article therefore, focuses on the attempts to introduce the topic of crystallography at GCSU, in an affordable way, for the past 3 years via the use of two new in-class activities.
2. Materials
- Two software suites were utilized for the design and implementation of these activities, (a) Mercury and (b) CrystalDiffract. From here on, the activities that use these software will be defined as (a) and (b).
2.1. Mercury – Crystal Structure Visualization [7]
- Mercury is a program freely available and published by the CCDC. This powerful crystal structure analysis software includes a “pay to use” portion of it, but also a free software which can be used for the analysis of .cif files (crystallographic information files) and other formats commonly found in crystallography (e.g. .res, .ins). The program can also be used to produce high quality images of molecules from the crystallographic data through POV-Ray rendering. The program is marketed as a powerful visualization tool, yet it has much more capabilities than that. Mercury can calculate PXRD patterns out of .cif files, visualize extended unit cells, calculate secondary interactions, and provide information on bond lengths/angles.
2.2. Crystal Diffract
- CrystalDiffract is a purchasable software suite which is published by CrystalMaker Software®. Though “pay to use”, it is one of the more affordable ones ($250 at the moment of this writing is the price for a personal license) and the company offers several licensing types. For this work, the personal license was used only by the author with awareness of terms and conditions for use. CrystalDiffract is just one of the many suites that the company offers and it is a PXRD analysis suite.
3. Methodology
3.1. Student Background
- The activities were implemented in the inorganic chemistry laboratory classroom and the advanced inorganic chemistry classroom. The audience consisted of a mixture of juniors, seniors, and science majors other than chemistry (e.g. biology). Activity (a) was implemented at the laboratory level, and activity (b) was implemented in the advanced inorganic chemistry classroom. At GCSU, the Inorganic Chemistry course is a requirement of the major, but the laboratory component is not (students are given the option to take other laboratories). The laboratory can be taken concurrently or after taking the regular course offering. Typically, only chemistry majors take the course, but the offering is available to other majors (e.g. biology) that meet the prerequisites/requisites. The advanced inorganic chemistry students had taken the intermediate inorganic chemistry course previously, but not necessarily the laboratory component. At GCSU, Advanced Inorganic Chemistry is considered one of the capstone courses required for graduation.
3.2. “Pet Molecule” – Introduction to Single Crystal X-Ray Diffraction Using Mercury
- Two laboratory sections (workshops) were dedicated to this activity. The first workshop was dedicated to introducing basic crystallography concepts: history, theory, and applications. Students were also instructed in the process of solving a simple crystal structure, from data collection to final refinements, and the output files resulting from the resolution (e.g. the .cif file, .res file, and .ins file). X-ray diffraction instrument vendors (such as Bruker or Rigaku) usually provide access to online webinars which instructors can use as part of a class. These can also be used to introduce students to crystallography. Figure 1 shows a typical image rendered in Mercury.
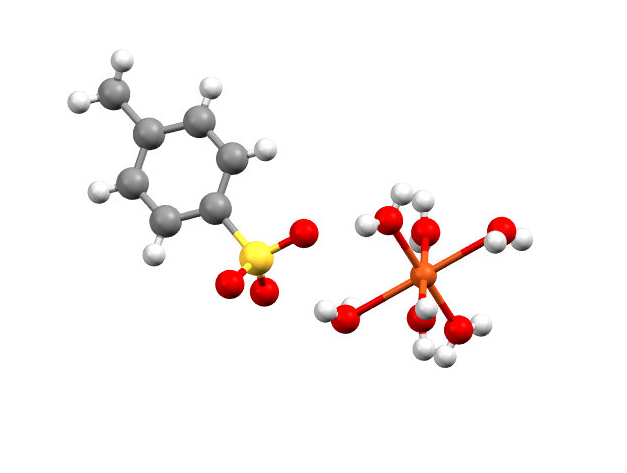 | Figure 1. Rendered image of a molecule in Mercury |
3.3. “Components in a Mixture” – A CrystalDiffract Powder XRD Activity
- The typical course layout of our advanced inorganic class consists of weekly topics, one of which is crystal structures. Much like activity (a), a class section was devoted to discussing history, theory, and applications of crystallography. In the second class, PXRD was introduced as an alternative to SCXRD if no single crystals are available (analysis of micro crystallites). Thus, the main objective of the activity was to expose the students to one of the advantages of PXRD over SCXRD, which is phase determination, and the determination of components in a mixture. The activity consisted of two parts, in the first part students were tasked to determine the identity of their 3 components. In the second part students were given a series of independent patterns where they had to employ Bragg’s Law (1) to calculate d-spacing. Where n is an integer from 1…∞, λ is the wavelength of the source (For PXRD, CuKα = 1.54 Å), d is the inter-planar distance between atoms in the crystal, and θ is the diffraction angle of the X-rays.
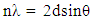 | (1) |
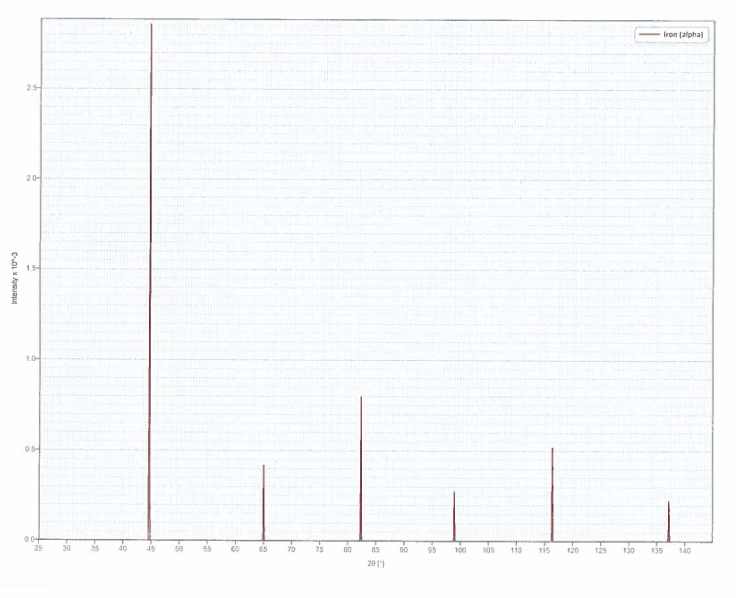 | Figure 2. Example of a CrystalDiffract PXRD pattern (Iron-α) |
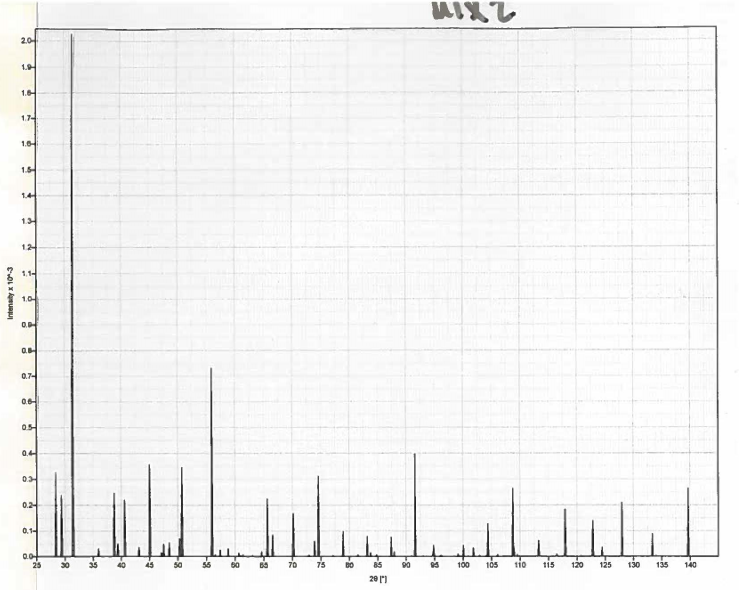 | Figure 3. An example mixture generated from CrystalDiffract |
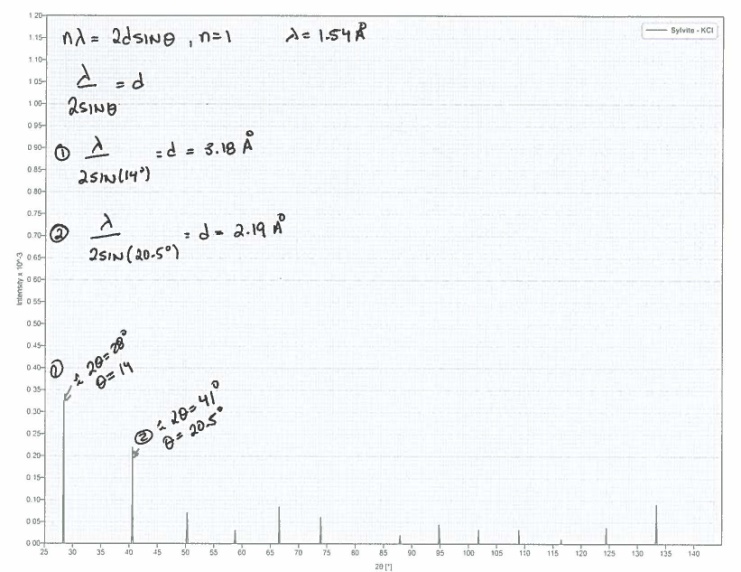 | Figure 4. Example calculation using Bragg equation in the sylvite PXRD generated pattern |
4. Discussion
4.1. Student Engagement
- For activity (a), student response was generally positive. For (a), ~85% of students (N = 48) scored above 40 (out of 50 points total), and had no difficulty using the software for their assigned molecules (students were also provided with plenty of time to complete the assignment). For activity (b), ~80% (N = 12) of the students successfully determined the assigned mixture compositions by overlaying (much like in a computerized database) the independent patterns over the assigned mixtures. In addition, 100% could apply Bragg’s Law to determine the d-spacing of 3 simple independent patterns (fluorite, diamond, and sylvite). Further, students understood that in order to use the PXRD angle data, they needed to convert 2θ to θ (2θ relating to the instrument). Also, students recognized the relationship between d-spacing size, 2θ of the instrument, and the θ of the Bragg equation (e.g. as 2θ increases, d-spacing decreases).
4.2. Instructor’s Notes
- The author believes that both activity (a) and activity (b) are highly customizable, and therefore highly adaptable to any PUI. For activity (a), all students had access to the Mercury software, which is freely available. Also, in the CCDC website, there are other resources that one can use if one desires to expand the activity. Recognizing that Mercury does have a “pay to use” version with extra features, if an instructor wishes to assign activity (a), then the free version of the program is sufficient for it. For (b), a personal license limits the use of the software to only the instructor. This is enough to generate content and use in a classroom environment. However, if a particular instructor would want a whole class to participate in PXRD pattern generation or other software capabilities, then a site/group license is recommended. It is important to note as well, when generating the simulated patterns, that instructors must keep note to be consistent on the scale of the y-axis and the x-axis (keep the same scale for all patterns to be able to overlay effectively). Finally, with some creativity, instructors can include topics such as unit cell determination, and information on crystallite size to their activity.
5. Conclusions
- Undergraduate student engagement in crystallography has been successfully implemented for the past 3 years at GCSU, within the inorganic chemistry courses. The two activities noted on this work are accessible to instructors at PUIs. Activity (a) uses Mercury- Crystal Structure Visualization software as its main tool to evaluate molecules chosen by students (“Pet Molecule”) and is freely available. Activity (b) uses CrystalDiffract as its main tool to determine the composition of mixtures and understanding PXRD, and is available at a modicum price. Both activities can be customized and modified to meet class needs.
ACKNOWLEDGEMENTS
- The author would like to acknowledge the Department of Chemistry, Physics, and Astronomy at GCSU for funds to purchase the CrystalDiffract suite. The author would also like to acknowledge the students who so graciously participated in the activities described here, and were instrumental in the generation of this report. Powder patterns were generated using CrystalDiffract®: a crystal and molecular structures program for Mac and Windows. CrystalDiffract Software Ltd, Oxford, England (www.crystalmaker.com).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML