-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
p-ISSN: 2331-7450 e-ISSN: 2331-7469
2018; 6(2): 24-30
doi:10.5923/j.jlce.20180602.02

A New Model for Advanced Undergraduate Chemistry Laboratory Courses: A Two-semester Integrated Laboratory Experience
Robert Haining, Joseph C. Sloop
School of Science and Technology, Georgia Gwinnett College, 1000 University Center Lane, Lawrenceville, GA, USA
Correspondence to: Joseph C. Sloop, School of Science and Technology, Georgia Gwinnett College, 1000 University Center Lane, Lawrenceville, GA, USA.
| Email: |  |
Copyright © 2018 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Chemistry faculty in the School of Science and Technology (SST) at Georgia Gwinnett College (GGC) have instituted a new two-semester laboratory capstone course sequence as part of the Chemistry major program. These integrated laboratory courses emphasize a multidisciplinary approach that provides a unique undergraduate experience for students. Integrated Laboratory I (Chemistry 4701 (IL-I)), which incorporates biochemistry-based laboratory techniques, practices and instrumentation, places students in a research-like setting similar to that found in graduate school. Integrated Laboratory II (Chemistry 4702 (IL-II)), which leverages organic qualitative analysis, synthesis and spectroscopic methods, affords students an understanding of chemical industrial remediation operations. This work describes the design, execution and success of these laboratory experiences. Student attitudinal surveys and course outcome assessments suggest this course sequence strengthens student critical thinking and problem-solving abilities toward answering questions of biochemical and chemical interest.
Keywords: Biochemical research, Undergraduate research creative experience (URCE), Organic synthesis, Spectral methods, Science, Technology, Engineering & math (STEM)
Cite this paper: Robert Haining, Joseph C. Sloop, A New Model for Advanced Undergraduate Chemistry Laboratory Courses: A Two-semester Integrated Laboratory Experience, Journal of Laboratory Chemical Education, Vol. 6 No. 2, 2018, pp. 24-30. doi: 10.5923/j.jlce.20180602.02.
Article Outline
1. Introduction
- As the Office of Professional Training for the American Chemical Society [1] has noted, undergraduate students majoring in the chemical sciences planning to enter graduate programs of study or those seeking employment in industrial research require a diverse skill set to be competitive for those opportunities. Skills-based chemistry program outcomes that require students to have a firm conceptual foundation in the theory, operation and maintenance of different analytical, spectroscopic and preparative laboratory instrumentation as well as their application in an undergraduate research experience are essential for success. In addition to these “hard” skills, are the expectations that students can apply critical thinking and problem solving to design and implement research plans, analyze data obtained from their work, and present their findings both orally and in writing with technical proficiency.While upper level undergraduate students can develop many of these skills by participating in typical capstone research courses, incorporating research throughout a student’s undergraduate program is a better model for ensuring that they have the requisite skills needed to succeed in industry or graduate school. For the past several years, the School of Science and Technology (SST) at GGC has participated in the University System of Georgia’s STEM II initiative [2] by implementing a very successful STEM four-year undergraduate research creative experience (4-Yr URCE) offered in forty-one courses [3]. Beginning at the introductory course level and continuing throughout their undergraduate studies at GGC, students in STEM disciplines engage in a variety of URCE projects [4]. Studies conducted by the National Research Council [5] and Lapatto [6, 7] indicate that students engaged in research in such integrated experiential learning settings throughout their entire STEM undergraduate program of study ensures that their senior-level research experience and internship opportunities are far more productive. Indeed, the positive effect of these URCEs may extend beyond graduation to graduate studies or employment [8]. The new Integrated Laboratory sequence (IL-I and IL-II courses) dovetails nicely with SST’s 4-Yr URCE program with one small distinction – the entire course sequence is taught in the laboratory. We engage undergraduate students in experiential learning that successfully melds critical thinking and problem-solving with research project design, advanced experimental technique application and instrumentation use in small-scale interdisciplinary research-like opportunities one might expect to find in graduate programs as well as those which emulate research in an industrial setting.The Integrated Laboratory-I course is a biochemistry-focused research experience similar to what students might expect to find in a graduate school setting, while the Integrated Laboratory-II course is a venue in which students apply advanced organic qualitative and spectral analysis protocols to identify unknown organic waste materials as members of a chemical remediation company. This paper examines the development of the Integrated Laboratory course sequence, methods used, instrumentation employed, and provides an initial evaluation of the course sequence success.
2. Integrated Laboratory Course Development
2.1. Backwards Design from Course Outcomes
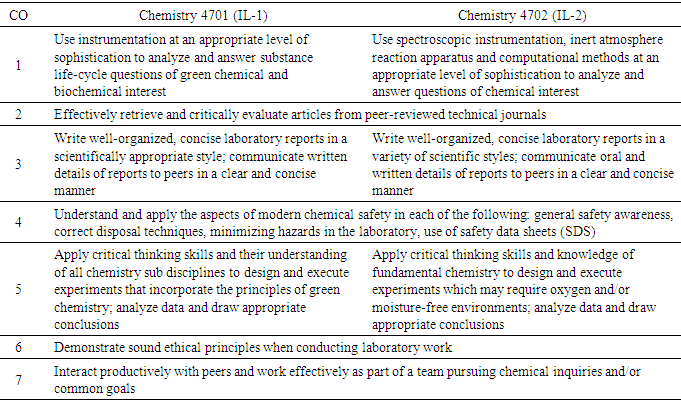
- Because these courses are capstone experiences, only students who met specific course pre-requisites and co-requisites were enrolled. For IL-I, these include both CHEM 3000K (Instrumental Analysis) and BCHM 3100K (Biochemistry I). For IL-II, students must have successfully completed IL-I and either have satisfactorily completed or be currently enrolled in CHEM 4201K (Physical Chemistry I). Taking these requirements into account and using a backwards design approach [9], faculty members selected to teach the Integrated Laboratory courses developed each course based on the course outcomes (COs) [10] shown in Table 1.
|
2.2. IL-I Course Layout and Requirements
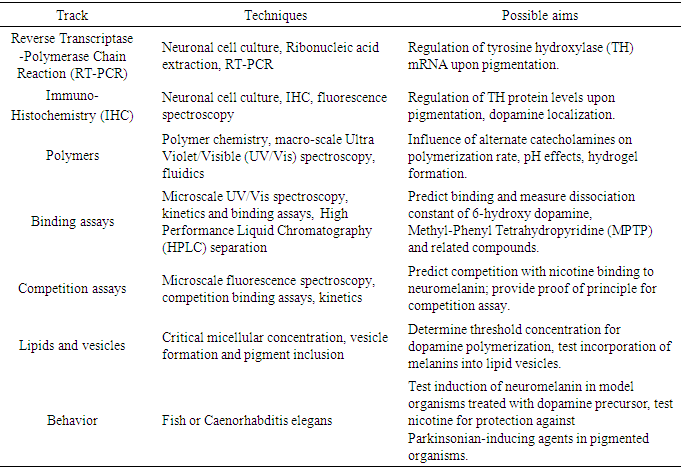
- Owing to the success of the 4-yr URCE model being implemented at GGC, students enrolled in IL-I were treated from the beginning of the course as if they were graduate students entering the laboratory with an expectation of independent thought and action. Rather than being handed specific protocols, students were informed, through reading and discussion early in the course, of the nature of the problem and of the overarching aims of the laboratory, with the instructor serving as Principal Investigator of a large grant. Their first assigned task was to choose from among several possible research pathways or tracks under the overarching hypothesis and develop a set of specific aims toward this goal using guidance from the National Institutes of Health (NIH) [11]. They were challenged with finding protocols using their own literature and internet search skills (CO 2). As a starting point for their literature search, a set of twenty core manuscripts was placed into the GGC MyCourses D2L-Brightspace learning management system online course content folder [12]. The research problem chosen for IL-I and presented to the students allowed for maximum flexibility and use of techniques in both chemistry and biochemistry. The overarching hypothesis involves the pigment neuromelanin (NM) which forms spontaneously in the brain from the cyclization of dopamine to compounds such as 5,6-dihydroxyindole and their subsequent polymerization. See Figure 1.
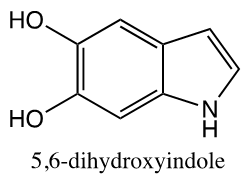 | Figure 1. Monomeric Component of Neuromelanin |
|
2.3. IL-II Course Layout and Requirements
- Students are welcomed to the course by the Chief Executive Officer (CEO) (instructor) of the Forensichem Chemical Remediation Corporation. In advance of chemical operations in the laboratory, students are provided with documents on the GGC MyCourses D2L-Brightspace online course content page that outline the company’s mission statement, analytical techniques, company capacities, reporting responsibilities, and disposition recommendations. Students are “hired” as bench chemists who are responsible for identifying unlabeled organic waste materials found in an abandoned chemical storage site which the company has been contracted to remediate. Students sign a non-disclosure agreement and are issued company laboratory notebooks that remain the property of the company. For the course, the unlabeled organic waste materials bench chemists would identify using both classical chemical testing methods and spectroscopic techniques, were, in fact, a large library of organic compounds assembled and placed in numbered vials [14]. In keeping with “green” chemistry principles, chemists were only provided with small quantities of the “unknowns” – usually less than ten grams. The compounds for the library were selected so as to have a broad array of solids and liquids containing aliphatic, unsaturated and/or aromatic components. Additionally, analytes were chosen that had a wide range of functional groups. See Figure 2.
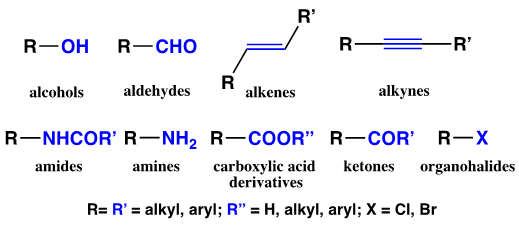 | Figure 2. Functional Groups Present on IL-II Unknowns |
|
3. Course Execution – Results and Discussion
3.1. IL-I
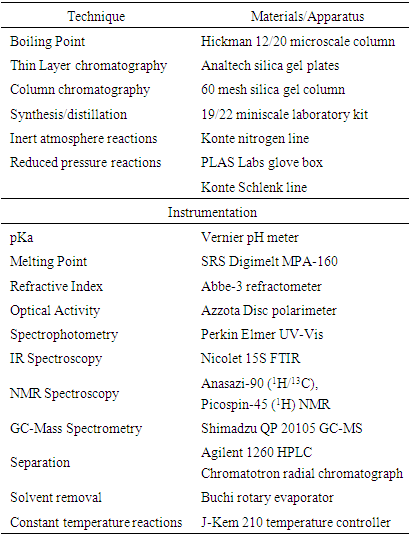
- In the first semester that IL-I was offered, three students registered for the course and chose the following research projects: HPLC analysis of dopamine and caffeine binding to NM, RT-PCR of pigmented vs non-pigmented cells, and fluorescence spectroscopy of amines in the presence of NM. In the second term, eight students registered for IL-I. To aid in scale up due to class size, students were assigned to work in pairs rather than individually. Students were still expected to produce independent specific aims and conclusions at the end of the term, but in this case, those with the most similar projects had a dedicated partner to work with and were asked to consult amongst themselves whenever possible to alleviate instructor time requirements. Projects chosen by IL-I students upon the second course offering built upon the data and conclusions of prior IL-I students and included: Influence of buffers and pH on melanin polymerization and the formation of hydrogels, investigation of competition binding against nicotine using nano-drop fluorescence spectroscopy, cell culture, RT-PCR, IHC, and effects of alternate catecholamines on melanin polymerization kinetics. Students were highly encouraged to follow interesting leads they encountered during their project to capitalize on serendipitous discovery. Indeed, one such discovery during the first IL-I term led to the development [17] of a nicotine binding assay with possible utility in measuring the binding constants of compounds which do not induce a unique spectroscopic signal of their own. This competition assay was then utilized by a pair of students in the following IL-I term, each choosing an individual compound for testing. In this manner, students were truly able to observe and benefit from the slow, steady progression of science in real time.
3.2. IL-II
- In the first semester that IL-II was offered, four students registered for the course and each selected four unknown organic compounds from among sixteen choices. In the second term, five students registered for IL-II. To aid in scale up due to class size, twelve more unknown organic compounds were added to the compound library to allow for rotation of compounds. All bench chemists successfully conducted their unknown analysis and derivatization briefing, identified their unknown compounds, synthesized their derivatives, and completed the reports and journal article as prescribed. Throughout the semester, students were encouraged to discuss their efforts with each other and post literature resources and protocols located via literature searches on the course D2L-Brightspace discussion boards. Additionally, students were assessed with three quizzes that measured their mastery of qualitative analysis methods, chemical testing mechanisms and spectroscopic analysis. During the first two terms, two students prepared 2,4-dinitrophenylhydrazones of aldehydes, two students prepared 2,4-dinitrophenylhydrazones of ketones, one student prepared a 2,4-dinitrophenylpyrazole, one prepared aspirin from salicylic acid, one prepared a carboxylic acid from an alcohol, one student prepared a tetrabromoalkane from an alkyne and one prepared an acetamide from an aromatic amine.
3.3. Success of the Integrated Laboratory Course Sequence
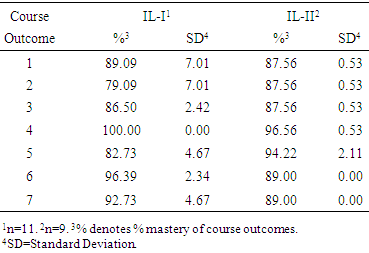
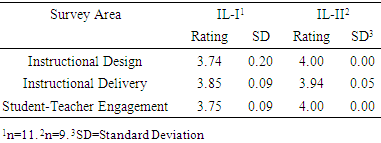
- Assessing the initial success of integrated laboratory course sequence was a function of several metrics. For IL-I, publication of scholarly products with student co-authorship related to the NM research project was a key indicator of the research value of the course. For both IL-I and IL-II, instructor-generated course assessment reports provided a measure of course outcome mastery. Finally, student end of course attitudinal surveys for the integrated laboratory provided a qualitative assessment of success with the courses in terms of instructional design and delivery as well as student-teacher engagement. Research conducted during the first two cycles of the IL-I course led to the preparation, submission and publication of two peer-reviewed articles. The first article, published in Neurochemical Research [18] featured GGC undergraduate students who participated in the integrated laboratory course. The second journal article, published in Neural Regeneration Research [13] featured GGC faculty who collaborated on neuromelanin research.As part of the overall Chemistry program assessment strategy, faculty teaching the integrated laboratory course sequence prepared course assessment reports following each semester. Evaluations of course success, based on the course outcomes (Table 2), formed a principal component of that assessment. Shown in Table 4 are the data for the first two cycles of the integrated laboratory course sequence.
|
|
4. Conclusions
- The first two cycles of the integrated laboratory course sequence were successful examples of how STEM students at Georgia Gwinnett College have benefitted from 4-Yr undergraduate research creative experiences. These courses provided unique opportunities for students to participate in projects and activities that mimic graduate research programs as well as chemical industry remediation operations. The open-ended laboratory-based nature of the courses creatively challenged students to use problem-solving skills to answer questions of biochemical and chemical interest. While future iterations of the course will doubtless see increased enrollment, new instructors, and different research opportunities for IL-I and chemical industrial applications for IL-II, the adaptability of the course sequence to new research directions will ensure its continued success as an integrated capstone research experience for GGC STEM majors.
ACKNOWLEDGEMENTS
- The authors thank Mr. Todd Wincek for chemical preparations and Dr. Sang Park for technical assistance with GC/MS operation.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML