-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
p-ISSN: 2331-7450 e-ISSN: 2331-7469
2017; 5(6): 129-134
doi:10.5923/j.jlce.20170506.02

An Experiment to Discuss Chemistry Effects on the Biological Behavior of Microorganisms
Wilmo Ernesto Francisco Junior
Campus Arapiraca, Universidade Federal de Alagoas, Arapiraca/AL, Brazil
Correspondence to: Wilmo Ernesto Francisco Junior, Campus Arapiraca, Universidade Federal de Alagoas, Arapiraca/AL, Brazil.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

An undergraduate laboratory experiment that can be used in Biochemistry, Toxicology and Microbiology courses to discuss the important effects of chemical compounds on metabolism is presented in this paper. The effects of pH and some metals (Hg, Mo and U) on ferrous ion oxidation by four Acidithiobacillus ferrooxidans strains were investigated using respirometry techniques. The biological oxidation of Fe2+ by the four strains is in agreement with the Michaelis-Menten kinetic model. The experiment can be an important tool to introduce this subject, as well as in opening possible discussions about other issues.
Keywords: Acidithiobacillus ferrooxidans, Iron oxidation, Lab work, Michaelis-Menten model
Cite this paper: Wilmo Ernesto Francisco Junior, An Experiment to Discuss Chemistry Effects on the Biological Behavior of Microorganisms, Journal of Laboratory Chemical Education, Vol. 5 No. 6, 2017, pp. 129-134. doi: 10.5923/j.jlce.20170506.02.
Article Outline
1. Introduction
- Acidithiobacillus ferrooxidans is one of the most important microorganisms used to recover metals from mineral sulfides. It is a gram-negative, acidophilic, aerobic and chemoautotrophic bacterium that obtains energy by the oxidation of inorganic substrates such as ferrous ion and reduced sulfur compounds, including metal sulfides. A. ferrooxidans is one of the few microorganisms that can obtain energy by oxidizing ferrous iron in acidic environments, using the low pH of its natural environment to generate reverse electron flow from Fe(II) to NADH. Due to its particular tolerance to metals, its pH and its ability to oxidize metallic sulfides, A. ferrooxidans has long been applied in industrial bioleaching operations for the extraction of copper, uranium and gold [1, 2].According to some authors [3, 4], a great alternative to improve the bioleaching of metal sulfides is to develop A. ferrooxidans strains able to maintain their biological activity in high metal concentrations. This is particularly important during the bioleaching process because the level of solubilized metals increases significantly. Thus, it is obvious that for the bioleaching process to be effective the bacterium must be resistant to the recovered metal as well as to the other metals in the environment. In this regard, many studies have demonstrated the influence of various metals on the growth and oxidation of ferrous ion by A. ferrooxidans. Several studies have reported that some metals are more toxic to the bacterium than others. Studies with uranium, thorium, cadmium, chromium, lead and silver have demonstrated their high toxicity to A. ferrooxidans, even at low concentrations [5-8]. On the other hand, metals such as Cu, Co and Ni have presented less toxicity to A. ferrooxidans [5-9].It is also likely observed that different strains of A. ferrooxidans will differ in their response to inhibitory metals, since some strains are more sensitive than others to certain metals [5, 6, 10, 11]. Furthermore, studies involving strains grown in the presence of heavy metals have demonstrated changes in the total protein synthesis patterns [11-13, 16]. This probably pertains to genetic aspects that establish a phenotypic variability between these strains, e.g., metal tolerance [12-16].Another aspect that influences biological behavior is pH. It is well known that variations in pH significantly modify the activities of both enzymes and microorganisms. Nonetheless, A. ferrooxidans strains can maintain their ability to oxidize metallic sulfides even in drastic conditions [1, 10]. During the bioleaching process, for example, pH decreases to levels between 1.0 and 2.0. Despite these unusual features, little discussion has focused on these aspects involving microorganisms in undergraduate courses. The unique features of A. ferrooxidans provide an interesting way to explore metabolism and the effect of chemical compounds on enzymatic activities.Based on the aforementioned discussion, knowledge about metal resistance, the influence of pH levels and the kinetic behavior of A. ferrooxidans on ferrous ion oxidation can lead to different learning possibilities. For this purpose, this paper presents an experiment to evaluate the kinetic oxidation of iron (II) by different A. ferrooxidans strains, as well the tolerance of these strains to variations in Hg, U and Mo and to pH in the range of 0.9-2.2. This study was performed by measuring O2 consumption coupled with ferrous ion oxidation through respirometric experiments.
2. Experimental Methods
2.1. A. ferrooxidans Strains and Growth Conditions
- Four A. ferrooxidans strains (LR, FG460, SSP and PCE) isolated from Brazilian uranium and coal mines were used in this work [17]. Isolation from acid mine drainage is the most common way to obtain the bacterium [17]. These isolates were grown in T&K medium [18] for 2 days at 30°C, with shaking (250 rpm). T&K medium is an aqueous solution for the optimum growth of A. ferrooxidans [18], and it is prepared from inorganic salts (K2HPO4.3H2O; MgSO4.7H2O; (NH4)2SO4, FeSO4.7H2O) at pH 1.8. The cells were then harvested by centrifugation (10,000 rpm) followed by 3 washes with H2SO4 solution at pH 1.8, and resuspended in 10 mL of acid solution (pH 1.8).
2.2. Respirometric Experiments
- Bacterial suspensions from four A. ferrooxidans strains were standardized before the respirometric experiments by determination of total nitrogen using the Kjeldahl method [19]. The experiments were conducted in a Warburg respirometer, using 25 mL Warburg flasks. The main compartment of each flask contained 2.0 mL of acid solution with oxidizable substrate. The cell suspensions (0.5 mL) were placed in the side arm of the Warburg flask and 0.1 mL of 20% (w/v) KOH in a Whatman number 1 filter paper was placed in the center well. In the experiments carried out in the presence of heavy metals, the acid solution contained 120 mMol L-1 of Fe2+ (substrate) as well as the following concentrations of metals (mMol L-1): U (1, 2, 4, 6, 8), Mo (2, 4, 6, 8) and Hg (0.1, 0.3, 0.5). Uranium, molybdenum and mercury, respectively, were used in the form of UO2(NO3)2.6H2O, Na2Mo4.2H2O, and HgCl2. Controls were also were carried out without added metal. The concentrations of ferrous ion used in the kinetic studies, were (mMol L-1): 2, 4, 6, 8, 10, 20, 40, 80, 120, 180, 240. The effect of different levels of pH: 0.9, 1.2, 1.8, and 2.3 was obtained by adding H2SO4 to the substrate solution. All the respirometric experiments were carried out in a water bath at 30°C and 150 strokes/min. Before 10 min had elapsed, the cells were added to the main compartment containing acid solution with metals and/or oxidizable substrate. Oxygen uptake was measured at 10 min intervals during the first 30 min of the experiment, and thereafter, at 15 min intervals. All the assays lasted 150 min and were performed in duplicate. The O2 uptake rate was calculated from the linear part of the curve. The results of our studies on the influence of the pH level and of heavy metals are expressed as relative values compared with the rate obtained in the control experiments of each A. ferrooxidans strain (without added metal and at optimum pH).
2.3. Hazards
- Although the chemicals used in this study are generally considered hazardous, the concentrations of the solutions were low. Nonetheless, care should be taken in their correct disposal, especially in the case of mercury and uranium. Mercury ions are usually precipitated by using hydrogen sulfide. However, this procedure generates a new waste product containing mercury that must be discarded in appropriate landfills. An alternative way is to reduce the Hg2+ ions by adding metallic zinc. The reaction is spontaneous and produces an amalgam. After the amalgam is formed, mercury can be removed by thermal desorption at 600-800°C, followed by the condensation of mercury vapors [20, 21]. In the case of uranium, anion exchange resins have been successfully employed to recover uranium [22, 23]. The use of mercury and uranium are not recommended if both professor and students have no work experience. In undergraduate courses, the use of metals other than mercury or uranium recommended in order to compare the results with those presented here for these metals.With regard to Acidithiobacillus ferrooxidans, it is a nonpathogenic species, but standard precautions such as eye protection, proper gloves, and lab coat should be used during the experiment.
3. Results and Discussion
- Respirometric experiments represent an indirect measurement of ferrous ion oxidation by means of oxygen uptake. This technique is suitable for this kind of investigation for different reasons, such as: i) the measures can be easily taken by students; ii) the experiments can be conducted relatively in a short time (each test can be ended in 90 minutes); iii) the principles of the technique involve important concepts from Physics, Chemistry and Biology, like pressure and pressure difference, allowing to explore interdisciplinary characteristics; iv) respirometric studies are widely employed in different researches on the biodegradation [24, 25]. From this, it is possible intertwining different areas, including historical aspects, taking into consideration that respirometric techniques are based upon very important physical-chemistry aspects that had also a fundamental role on the Lavoisier’s studies, from which the identification of the oxygen function in oxidation process was possible and redirected the Chemistry as a Science.
3.1. Kinetic Studies
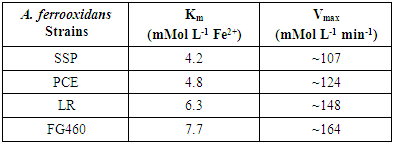
- The oxygen uptake calculated for each experiment was utilized to build plots of the effect of substrate concentration on the initial reaction rate. The results indicated that the iron II oxidation rate is consistent with the Michaelis-Menten substrate saturation kinetics (Figure 1), which has also been demonstrated by other authors [26, 27].
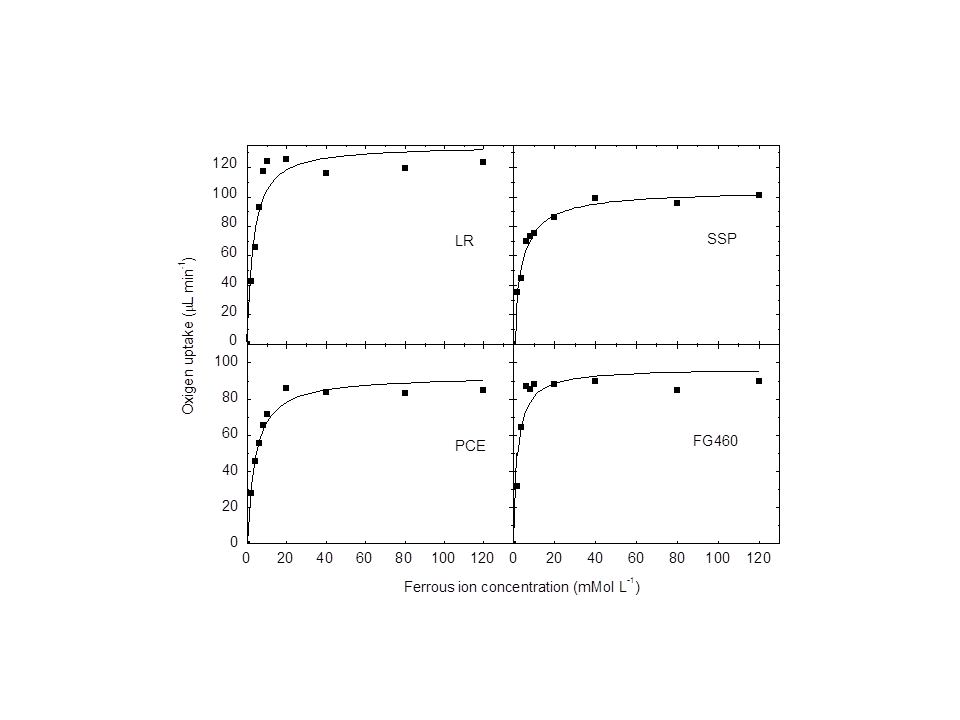 | Figure 1. Effect of ferrous ion concentration on the oxygen uptake of all the Acidithiobacillus ferrooxidans strains. (A) Af-LR, (B) Af-SSP, (C) Af-PCE, (D) Af-FG460 |
|
3.2. Effect of Heavy Metals on Iron Oxidation
- Figure 2 shows the effect of the metals mercury, molybdenum and uranium on the iron oxidizing ability of the four A. ferrooxidans strains under study. It is clear that different strains exhibit specific sensitivities to the tested metals. At the lowest concentration tested (0.1 mMol L-1), mercury (Figure 2A) exhibited very high toxicity (relative oxygen uptake lower than 45%) in all the strains. At concentrations above 0.1 mMol L-1, the inhibition was more evident. These results are in agreement with previous studies, which found that concentrations higher than 0.1 mMol L-1 cause deleterious effects on the iron oxidation and growth of A. ferrooxidans [28, 29], and stated that 0.005 mMol L-1 suffices to trigger an inhibitory effect [6]. The results confirmed that mercury was the most toxic metal tested. In the presence of uranium (Figure 2B), the LR strain was the most resistant, and was inhibited by second highest concentration (6 mMol L-1), while the PCE strain was less tolerant to uranium than LR. A. ferrooxidans LR was able to oxidize ferrous ion (75 and 46% respectively) at concentrations of 2.0 and 4.0 mMol L-1. At concentrations higher than 4.0 mMol L-1, the PCE strain was almost fully inhibited. Strains FG460 and SSP exhibited no significant activity in the presence of the lowest uranium concentration. The levels of uranium resistance obtained in this study are in general agreement with data reported in the literature [5, 29], which reach tolerance levels in the range of 2 to 8 mMol L-1.Among all the tested metals, molybdenum provided the best isolate differentiation for the A. ferrooxidans strains, as illustrated in Figure 2C. Molybdenum was considerably more toxic to the FG460 and SSP strains, which maintained low Fe2+ oxidizing activity (oxygen uptake relative lower than 40%) at all the molybdenum concentrations. At a concentration of 1 mMol L-1, the LR and PCE strains were slightly inhibited. At higher molybdenum concentrations (3 and 4 mMol L-1), the inhibitory effect of these isolates was more evident (> 90%). Similar findings have been reported by Pistaccio et al. [30], who demonstrated complete inhibition on the growth of A. ferrooxidans at 2 mMol L-1 Mo, and an initial inhibition at 1 mMol L-1.
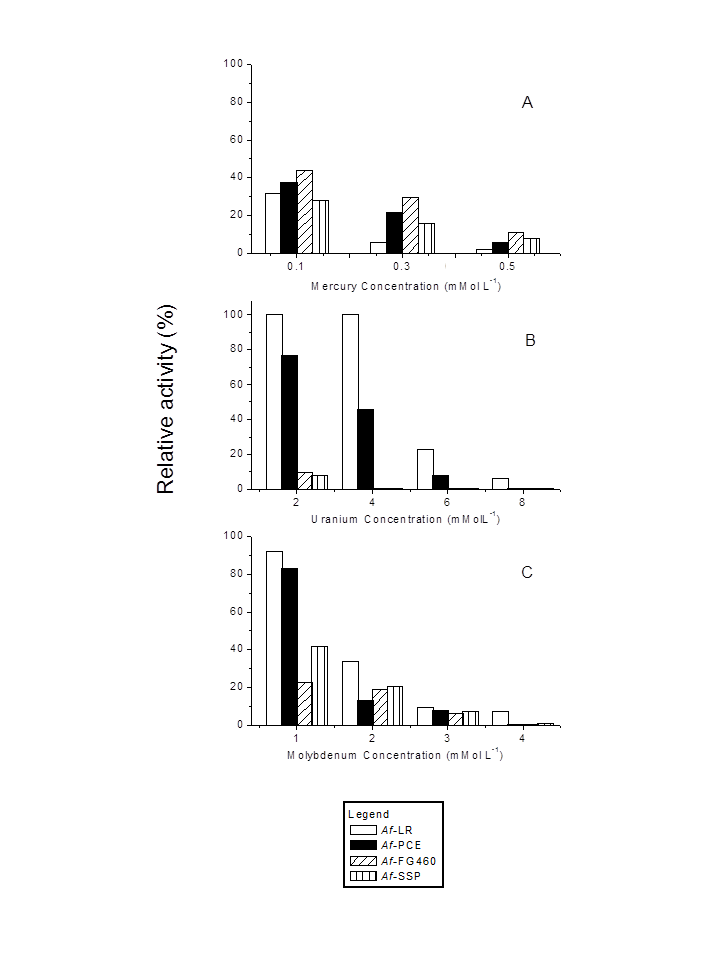 | Figure 2. Effect of mercury (A), uranium (B) and molybdenum (C) on ferrous ion oxidation by resting cells of four Acidithiobacillus ferrooxidans strains |
3.3. Effect of pH on Iron Oxidation
- The studies performed at different pH levels allowed to differentiate the A. ferrooxidans strains. Figure 3 shows that, except for LR, no strain was able to oxidize ferrous ion at the lowest tested pH level (0.9). Some studies have reported strong bacterial inhibition at pH levels of around 1.0 [27, 31]. A highly relevant aspect at a pH level as low as this one is the inhibition of bacterial activity through acidity, which probably involves the structure and composition of the cell wall or the cytoplasmic membrane, or both [27]. The increase in pH caused an increase in bacterial activity. At pH 1.2, strains LR and FG460 showed a relative oxygen uptake very similar to the optimum pH level (1.8). In contrast, strains SSP and PCE presented higher inhibition by pH, indicating higher sensitivity to acid than the other strains. At the highest pH level tested (2.3), all the strains presented high activity. The highest sensitivity at this pH level was detected in the PCE strain, which showed approximately 85% of relative O2 uptake.
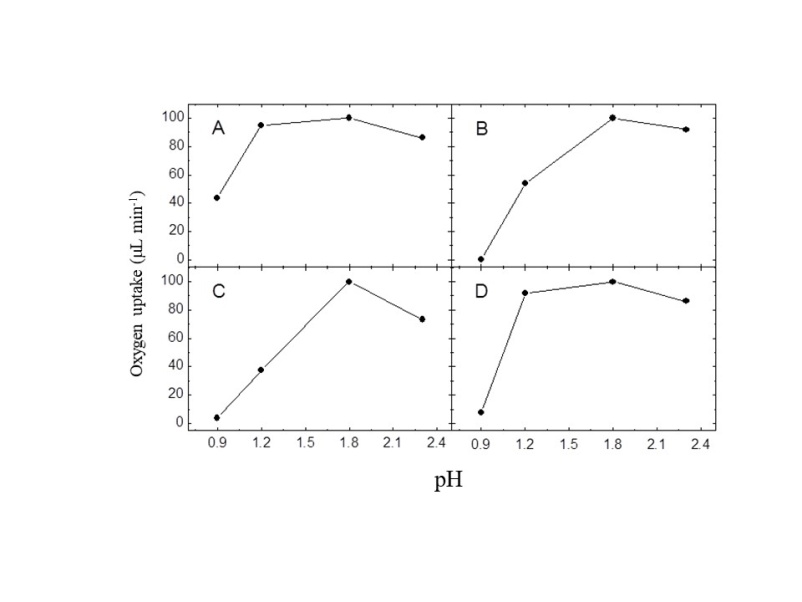 | Figure 3. Effect of pH on Fe2+ oxidation by resting cells of four Acidithiobacillus ferrooxidans strains. (A) LR, (B) SSP, (C) PCE, (D) FG460 |
4. Conclusions and Educational Implications
- This work does not aim to provide an experiment to illustrate enzyme kinetics, but rather, to propose a situation that can serve as a starting point to initiate discussions about different aspects. A positive aspect is that these experiments can be conducted in 90 minutes, a short time in terms of laboratory practices. The respirometric techniques are based upon very important physicochemical aspects that also play a fundamental role in History, such as Lavoisier’s studies. The principle is based on the pressure difference by means of oxygen uptake. Ferrous ion oxidation, which is catalyzed by bacteria into ferric ion, involves a process that consumes oxygen. The mechanism of iron oxidation involves the oxidation of ferrous iron outside the cell membrane at an acidic pH around 2.0 and the reduction of oxygen inside at a pH of 6.5, with the iron-oxidizing system requirement ions sulfate. Investigations [32, 33] have been characterized different proteins that can play an important role. The enzymes that have been isolated and characterized are rusticyanin, Fe(II)-cytochrome c oxidoreductase, cytochromes c-552(s), c-552(m) and c-550(m), and cytochrome c oxidase. This oxidative process is in agreement with the Michaelis-Menten model. In this context, the experiment can be carried out before offering a presentation of the Michaelis-Menten kinetics, in order to discuss the reasons why oxygen uptake reaches the steady state. The measurement of the initial O2 uptake enables to identify important parameters about the kinetics of the ferrous ion oxidation process. Hence, the experiment is an important tool to introduce this subject. However, the kinetic parameters obtained here are in disagreement with those reported by various other authors. These differences can be explored to discuss hypotheses put forward by students, as well as the divergences biological systems can show in terms of Michaelis-Menten kinetics.Some additional questions to be explored are: What metabolic characteristics can cause A. ferrooxidans to display this unusual behavior at low pH? What are the differences between photoautotrophic, chemoautotrophic and chemolithoautotrophic bacteria? What kind of biotechnological applications can be developed from the specific features of A. ferrooxidans? Similar experiments can be also conducted using different microorganisms and chemical effects to study different behaviors. Based on the results, it is possible to establish an order of metal and acid toxicity for each culture. In terms of metal resistance, the different isolates presented different patterns in response to each tested metal. Mercury was the most toxic metal, inhibiting all the strains even at the lowest concentration tested. With regard to molybdenum and uranium, we were able to differentiate the most resistant strain, an found that LR was the strain most tolerant to these metals. From the standpoint of pH, the LR strain also exhibited a better behavior, since it showed oxidative activity at all the tested pH levels.Although the experiments were performed with a Warburg respirometer (a manometric apparatus), other similar apparatuses can be employed for the same purpose. The interest of this work lay in a quantitative study aimed at determining some kinetic parameters. However, simpler experiments can be conducted in beakers by visually analyzing the color resulting from iron oxidation. The duration of these experiments is very similar. These experiments allow qualitative aspects to be explored and the same results to be achieved with regard to comparisons of resistance to metals and to pH. Experiments can be performed using a variety of microorganisms, metals and pH levels to compare their metabolism and to enrich learning, since the biological behavior of these microorganisms may differ. The unique features of A. ferrooxidans are an interesting theme of discussion with students. While most biological organisms use organic compounds to obtain energy, this bacterium is able to oxidize specific inorganic substrates, such as reduced forms of iron and sulfur, enabling it to survive in aggressive chemical environments. This tendency of A. ferrooxidans can lead to other possible themes for study, including hydrometallurgical operations and genetic studies.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML