-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
p-ISSN: 2331-7450 e-ISSN: 2331-7469
2017; 5(5): 116-119
doi:10.5923/j.jlce.20170505.03

A Laboratory Demonstration for the Estimation of the Percentage of Oxygen in Air
Fan Yang, Zhen Zhang, Cheng-yin Yang
School of Chemistry & Chemical Engineering, Shaanxi Normal University, Xi’an, China
Correspondence to: Cheng-yin Yang, School of Chemistry & Chemical Engineering, Shaanxi Normal University, Xi’an, China.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This paper discussed a method to improve the estimation of the oxygen content in the atmosphere. An indicating solution containing methylene blue (MB), glucose and other chemicals was used to detect unreacted oxygen in residue gas after the combustion of white phosphorous. The optimized MB-glucose solution verified that the amount of remaining oxygen after combustion was significant. The amount of unreacted oxygen was accurately measured by polarography and gas chromatography. Subsequently, the corrected value of the oxygen content from this experiment was obtained by adding the amount of unreacted oxygen. Results showed that the adjusted value was close to the theoretical value.
Keywords: White phosphorus, Air, Oxygen, Methylene blue, Polarography, Gas chromatography
Cite this paper: Fan Yang, Zhen Zhang, Cheng-yin Yang, A Laboratory Demonstration for the Estimation of the Percentage of Oxygen in Air, Journal of Laboratory Chemical Education, Vol. 5 No. 5, 2017, pp. 116-119. doi: 10.5923/j.jlce.20170505.03.
Article Outline
1. Introduction
- The measurement of oxygen content in the atmosphere is one of the fundamental experiments for the primary education of chemistry. This experiment traditionally uses a candle or vigorous combustion reactions inside a closed container that is partially immersed in water. These reactions have striking characteristics, thus making them easy to demonstrate in a classroom setting. However, the “candle-and-cylinder” test for measuring the changing amount of oxygen content has limitations such as the thermal expansion of the gas phase and low oxygen consumption [1, 2]. Thus, the oxygen content in the volume fraction of some experiments is always lower than the theoretical value (21%) [3, 4]. Chemical educators have been seeking better alternatives to improve this measurement [5, 6]. The use of quantitative equipment in measuring oxygen content has also been discussed [4, 7, 8]. Traditional combustion experiments can be an impressive class demonstration if the amount of unreacted oxygen can be computed accurately.Methylene blue (MB) solution, which is highly sensitive to oxygen gas, can be used as a possible indicator to detect small amounts of oxygen. The main chemical components of this solution are MB and glucose. Campbell et al. [9, 10] first reported the MB–glucose–oxygen system by the “blue bottle” experiment. The reactions involved in the MB–glucose–oxygen system are as follows:
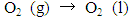 | (1) |
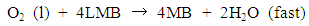 | (2) |
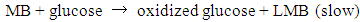 | (3) |
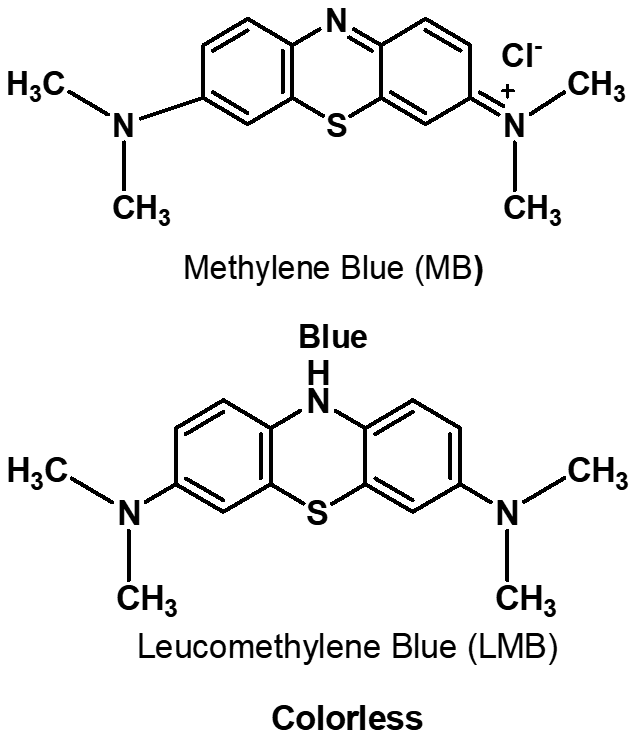 | Figure 1. Structures of methylene blue (MB) and leuco-methylene blue (LMB) |
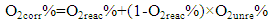 | (4) |
2. Experimental
2.1. Hazards
- White phosphorus causes burns and is highly toxic by ingestion, inhalation, and skin absorption. Keep it under water and away from heat when not use. White phosphorus can combust upon exposure to oxygen spontaneously and product tetraphosphorus decaoxide, which is also extremely corrosive and toxic. Furthermore, the combustion of white phosphorus should be performed in a fume hood or with proper ventilation. Having a fire extinguisher is necessary while performing this demonstration.It is safe to use methylene blue and glucose if used prudently. Avoid exposure to skin or eyes. If they contact skin or eyes, wash with copious amounts of water for at least 15 minutes and get medical aid. The sodium hydroxide and its concentrated solution are caustic chemicals that burn skin on contact. Thus, protective laboratory equipment (gloves and goggles) is required when handling these substances. (Further details are included in the supporting information.)
2.2. Combustion
- A 50 mL glass syringe, which was sealed with a rubber conduit at the top, with a movable piston, was used for the combustion of white phosphorus (Figure 2). Approximately 1 g of white phosphorus was added into the syringe cylinder. After that, the piston of the syringe was maintained at a scale of 40 mL, and the tip of the syringe was dipped in a hot water bath (>60°C). The syringe was removed from the water bath the moment the white phosphorus starts to burn. The syringe was fixed on an iron stand during the combustion process. A controlled trial was performed by following the same procedure and gently shaking the syringe during the reactions. After the combustion process and the syringe reached the room temperature, the amount of consumed oxygen was estimated by reading the different positions of the piston. The residue gas was then carefully transferred into another syringe filled with 1 mL indicator solution as shown in Figure 3. An appropriate indicator solution was prepared by combining glucose, NaOH, and drops of MB solution.
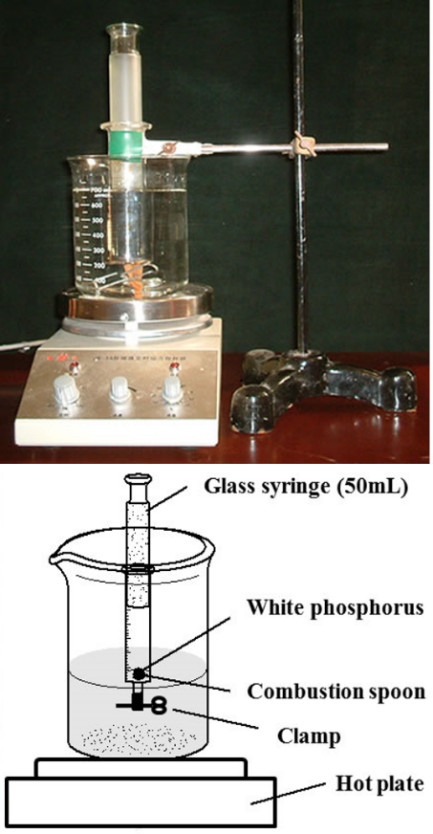 | Figure 2. Experimental device for determining the oxygen content in air |
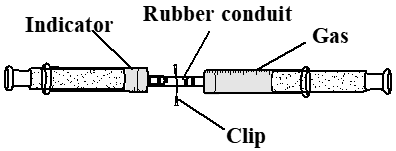 | Figure 3. Device for determination of residual air by the indicator solution |
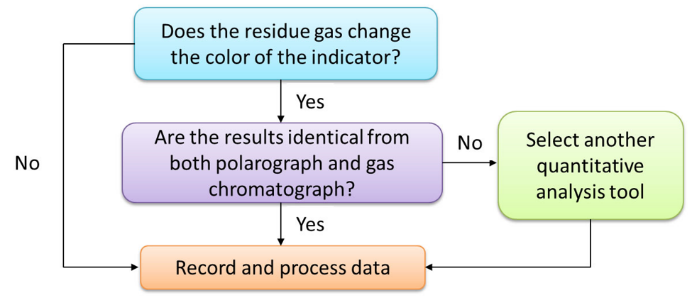 | Figure 4. Logic tree of estimation the unreacted oxygen |
2.3. Oxygen Measurement by Polarograph and Gas Chromatograph
- KY-2B oxygen controlled polarographic analyzer (Meicheng Instrument, China) was employed in this experiment to detect a trace amount of oxygen. Agilent-6820 gas chromatograph (Agilent. USA) was also used. The carrier gas was the high purity helium (99.99%), and the GC column was molecular sieves 5A (Restek. USA). Ultra-high purity oxygen gas (99.999%) was worked as a reference.
3. Results
3.1. Optimization of Indicator Solutions
- Figure 5 shows the time of blue color fading away as a function of NaOH and glucose concentrations. The concentration of NaOH and the fading time has a power function relationship while the concentration of glucose solely influenced the fading time. Furthermore, the kinetic research indicates this reaction rate is almost first order in the concentration of NaOH and independent on the concentration of glucose [12, 13]. When the concentration of NaOH is larger than 0.1 M, their fading time proceeds over half an hour, and it is too long for this color test. Similarly, when the glucose concentration is above 1.0 M, the color of the solution is unclear yellow-brown, which impedes the study of color. From this practical view, these concentrations of both NaOH (>0.1 M) and glucose (>1.0 M) are not suitable for making test solutions. Therefore, the appropriate indicator solution is prepared by the mix of the solution of glucose and NaOH (CGlucose : CNaOH =0.5 M: 0.5 M) and a few drops of methylene blue solution, and its fading time only takes a few minutes.
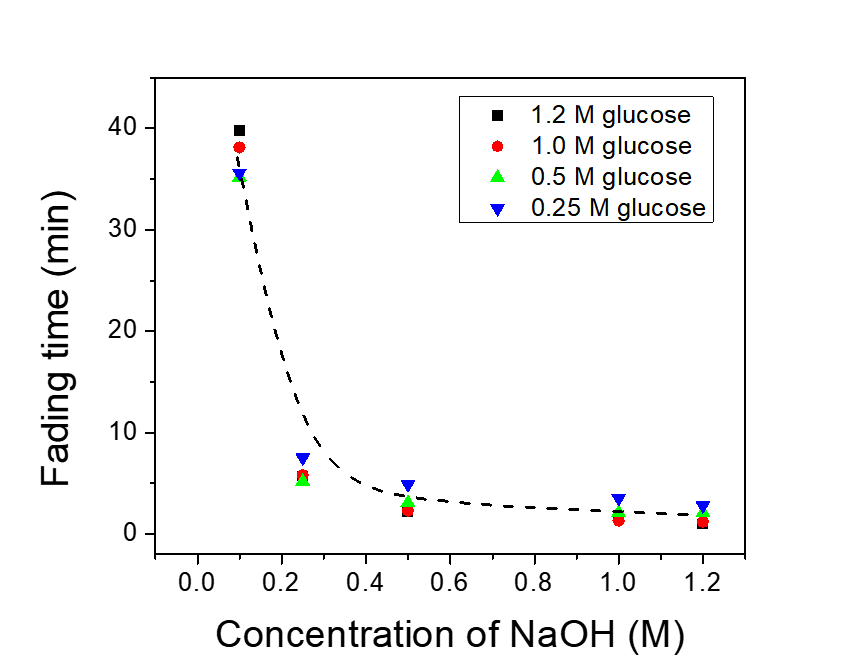 | Figure 5. Indicator fading time as a function of the NaOH concentration |
3.2. Quantitative Analysis of the Unreacted Oxygen
- The detailed discussion of residue oxygen measurement is in the supplemental information. Measurements results of unreacted oxygen by polarograph was carried out by fixing the syringes on the iron stand and by shaking the syringe. As a result, the oxygen contents of two methods are 4.6±0.7% and 1.3±0.4%, respectively.The GC result of the unreacted oxygen contents from two different methods (holding or shaking the syringe) is 4.6±0.7% and 1.3±0.4%, respectively. We approach the measurement results from two quantitative instruments. After corrected by adding the unreacted oxygen to the directly reading, the oxygen contents by two handling methods are close (Table 1).
|
4. Discussion
- The experiment in this study is designed for middle school or high school chemistry demonstrations. However, only the combustion of white phosphorus in syringes and the MB indicator test can be performed during class. The quantitative analysis of unreacted oxygen is hard to illustrate because of its complexity and time-consuming nature. Thus, the teacher can use unreacted oxygen data only to calculate the adjusted oxygen content in the air and compare the obtained value with the theoretical value.Two main issues cause errors in oxygen content measurements: (i) thermal changes occur in the limited volume expulsion of air; (ii) combustion does not consume all oxygen in the system. The products of white phosphorus combustion are solids rather than gases, which do not interfere with the volume of the depleting oxygen. In this experiment, glass syringes are used as the reaction container instead of vessels in water because glass syringes are more accessible for volume measurement and for cooling to room temperature. This improvement in the apparatus overcomes the disadvantage of thermal expansion during reactions. The oxygen content measured from the combustion of white phosphorus is less than the theoretical value, thus indicating that partial oxygen is unreacted in the system. Given that this combustion process is a heterogeneous reaction (solids in gases), the reactants do not react entirely because of insufficient exposure to each other. Shaking the syringe during the combustion process can assist white phosphorus to react sufficiently with oxygen. The oxygen content measured by this method is approximately 20%, which is closer to the theoretical value of oxygen content (21%) than the result (about 17%) from the standing syringe. However, complete combustion cannot be achieved by only shaking the syringe, and the effect of this method is acceptable only in class demonstrations. The indicator tests signify the presence of residual oxygen after the combustion reaction from both methods, thus indicating that the analysis of unreacted oxygen is necessary. The assessment results show that the adjusted oxygen content in the air is identical with that of the theoretical value.
5. Conclusions
- A syringe with a piston was used as an alternative reaction device to improve the discovery of oxygen contents in the air after white phosphorus combustion. Shaking this apparatus during the reaction was an efficient method to obtain oxygen content close to the theoretical value. Although residual oxygen was detected after the reaction by a MB-glucose indicator, the preliminary data could be further adjusted by adding the correct amount of unreacted oxygen, which determined by the quantitative analysis.
ACKNOWLEDGEMENTS
- The authors are grateful to Mr. Ai-Wu Shi, who offered expert assistance in the gas chromatogram analysis. This work is supported by the research project of Teachers’ Education of Shaanxi Normal University (construction of the curriculum system aiming at cultivating outstanding teachers of science. No. JSJY2015J009).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML