-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
p-ISSN: 2331-7450 e-ISSN: 2331-7469
2017; 5(4): 86-93
doi:10.5923/j.jlce.20170504.04

Novel Demonstration of Heterogeneous Redox Catalysis using a Rotating Pencil Lead
Andrew Mills1, Rachel Andrews1, Christopher O'Rourke1, Michael Hitchman2
1School of Chemistry and Chemical Engineering, Queens University Belfast, Belfast, UK
2Department of Pure and Applied Chemistry, University of Strathclyde, Glasgow, UK
Correspondence to: Andrew Mills, School of Chemistry and Chemical Engineering, Queens University Belfast, Belfast, UK.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Heat-treated, pencil lead, in powder or rod form, is used as a heterogeneous redox catalyst for the oxidation of chloride to chlorine by Ce(IV) ions in 0.5 M H2SO4 and 2 M NaCl solution. When the lead is used in ground up powder form, the resulting decay of the Ce(IV) is first order over 3 half-lives and gives a chlorine yield of 79%. When a rotating 2 mm diameter, heat-treated form of the pencil lead is used in the same test system, the kinetics of Ce(IV) yields a series of different first order rate constants, k1, as a function of rod rotation speed, ω. A plot of k1 versus ω0.7 yields a good straight line, which accords with the hydrodynamics of the system and the assumption that the rate of Ce(IV) reduction, by chloride, depends upon the rate of mass transport of the Ce(IV) ions to the surface of the pencil lead rod. The gradient of the plot allows a value for the diffusion coefficient for Ce(IV) ions in 0.5 M H2SO4 and 2 M NaCl solution to be calculated, namely: (2.7 ± 0.1)x10-6 cm2 s-1) which compares favourably with those reported previously.
Keywords: Electrochemistry, Kinetics, Redox Catalysis, Pencil lead, Ceric ions, UV-Vis spectroscopy
Cite this paper: Andrew Mills, Rachel Andrews, Christopher O'Rourke, Michael Hitchman, Novel Demonstration of Heterogeneous Redox Catalysis using a Rotating Pencil Lead, Journal of Laboratory Chemical Education, Vol. 5 No. 4, 2017, pp. 86-93. doi: 10.5923/j.jlce.20170504.04.
Article Outline
1. Introduction
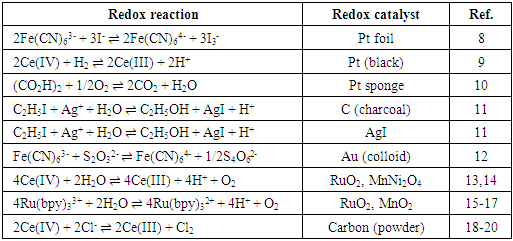
- Most physical chemistry textbooks [1-3] discuss the behaviour of single redox couples, such as Ox1/Red1,
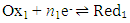 | (1) |
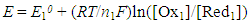 | (2) |
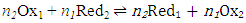 | (3) |
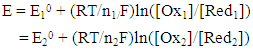 | (4) |
|
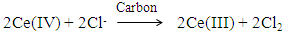 | (5) |
1.1. Theoretical Concepts
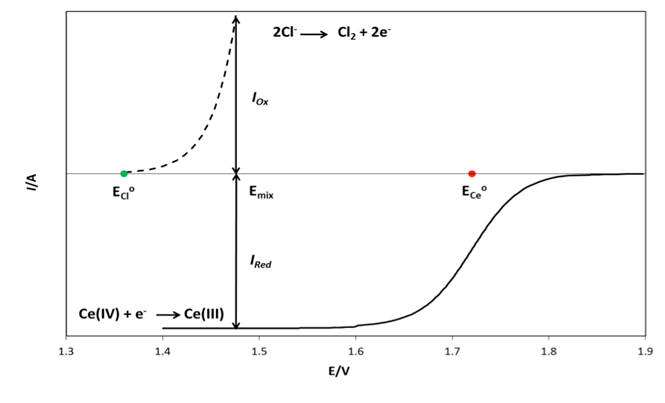
- Redox catalysis is routinely interpreted using an electrochemical model [5, 20, 26-28] in which the redox catalyst acts as an electrode, or micro-electrodes if particles are used, that conduct electrons between the two redox couples. In this model the observed kinetics of the overall redox reaction are due to the combination of the current-voltage curves of the two contributing couples on the redox catalyst. For example, in the case of the redox reaction (5), the Ce(IV)/Ce(III) couple is known to be electrochemically reversible; i.e. it exhibits a high exchange current density, io = ca. 50 mA cm-2, [29] when used with most conducting materials, whereas Cl2/Cl- is, in comparison, irreversible; io = ca. 0.25 mA cm-2 (on graphite) [30]. In addition, their standard redox potentials, i.e. ECeo and EClo, are 1.72 and 1.36 V, respectively, and so the two current-voltage curves are well separated [20]. As a consequence, the very different current, i (units: A) voltage curves associated with these two couples at the start of the reaction can be represented by those illustrated in Figure 1. The current-voltage curve for the oxidation of Cl- to Cl2 is typical for an electrochemically irreversible reaction, where the reactant, [Cl-] is very large ([NaCl] = 2 M in this work) so that the electrochemical reaction is always controlled by electrochemical-driven, surface reaction kinetics, rather than mass-transport, and thus is described by a Tafel equation; i.e.
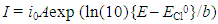 | (6) |
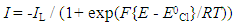 | (7) |
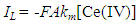 | (8) |
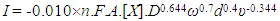 | (9) |
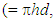 where h is the length of the exposed rod electrode of diameter, d (units: cm)), D is the diffusion coefficient for X (units: cm2 s-1), ω = rotation speed (units: rpm and ν = kinematic viscosity (units: cm2 s-1).In the case of reaction (5), the kinetics can be monitored via the disappearance of the [Ce(IV)] so as to yield a value for k1 for each selected rotation speed, ω. Under these conditions,
where h is the length of the exposed rod electrode of diameter, d (units: cm)), D is the diffusion coefficient for X (units: cm2 s-1), ω = rotation speed (units: rpm and ν = kinematic viscosity (units: cm2 s-1).In the case of reaction (5), the kinetics can be monitored via the disappearance of the [Ce(IV)] so as to yield a value for k1 for each selected rotation speed, ω. Under these conditions, 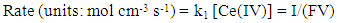 | (10) |
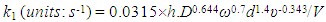 | (11) |
2. Experimental
- The 0.1 M Ce(IV) sulphate solution in 0.5 M H2SO4 (Sigma Aldrich) was either used as received (in the ground pencil lead experiment) or used to make up the 3.5x10-3 M Ce(IV) sulfate in 0.5 M H2SO4, plus 2 M NaCl, which was employed in the rotating pencil lead experiments. The 2 mm diameter KOH-I-NOOR 2H grade graphite pencil lead had the following weight % composition: w(graphite) = 0.60; w(clay) = 0.34 was purchased from Amazon and heated at 450°C for 30 min, prior to use, in order to burn off the wax (ca. 6 wt%) that is used as a coating. In the absence of this step the yield of Cl2 via reaction (5) is only 8%, but increases to 79% after heat conditioning. Presumably oxidisable adventitious impurities, in the clay or remnants of the wax, are responsible for the less than 100% yield of Cl2, since the pencil lead is only ca. 60% graphite; cf. earlier results mentioned above [18-20]. In order to confirm the efficacy of the graphite in the pencil lead as a redox catalyst for reaction (5), one of the pencil leads was ground up in a pestle and mortar, heated to 450°C for 30 min before being used to create a suspension (60 μg cm-3) of the redox catalyst in a solution, comprising 0.5 M [M] H2SO4 and 2 M NaCl, 2.5 cm3 and 0.4 mg cm-3 fumed silica (COK 84), which were placed in a 1 cm quartz cuvette, with a crown stirrer flea [36] and placed in a Cary 50 UV/Vis spectrophotometer, fitted with a magnetic stirrer. The fumed silica is an inert antifloculant, without which the carbon particles would tend to aggregate and become less effective as a redox catalyst [37]. The cell's contents were stirred continuously and the reaction was initiated via the injection of 90 μL of the acid 0.1 M Ce(IV) sulphate solution to the stirred catalyst dispersion. The instrument was set to monitor the absorption spectrum of the spectrophotometric cell and its contents at 1.5 min intervals, or to monitor the decay of the absorbance due to the Ce(IV) at 430 nm.In the wireless rotating pencil lead experiments, the heat-treated pencil lead was rotated using a 15 V DC electric motor (Rank Brothers Ltd., Cambridge, UK), the metal shaft of which was connected to a plastic sleeve which held the 2 mm pencil lead. The exposed cylinder to distal end area ratio was 61:1, suggesting that only ca. 2% of the observed kinetics were due to the rotating distal end, so that eqn (11) will be approximately true. In a typical experiment, a 1 cm empty quartz cuvette was placed in the sample chamber of the Cary 50 UV/Vis spectrophotometer, which was set to monitor the absorbance of the contents of the spectrophotometric cell at 430 nm as a function of time. The 2 mm pencil lead was then placed in the centre of the empty 1 cm quartz cuvette, so that 3.1 cm of the rod’s length would be exposed to the reaction solution and it was then rotated at the desired rotation speed (400-1200 rpm). The reaction was initiated by injecting 3.5 cm3 of the reaction solution, namely: acid 3.5x10-3 M Ce(IV) sulfate, plus 2 M NaCl, aqueous solution, into the cuvette and the decay of the Ce(IV) was monitored spectrophotometrically as a function of time for 10 min. This short monitoring time was chosen so that the whole experiment could be completed in 1.5-2 h; i.e. within an afternoon. All reactions were performed in a fume hood, so as to avoid operator exposure to the small amount of Cl2 generated (< 0.04 cm3 per run). All reactions were studied under ambient conditions at room temperature, 22°C.
2.1. Hazards
- Sulfuric acid and Ce(IV) sulfate are both corrosive. Avoid inhalation and skin and eye contact. If either is splashed into the eyes, rinse cautiously with water for several minutes. Remove contact lenses, if present, and easy to effect, and continue rinsing. Chlorine is an oxidising gas, which has acute toxicity if inhaled and may cause skin and eye irritation. Avoid inhalation and skin and eye contact. Carry out all experiments in a fume hood, wearing eye protection and gloves at all times.
3. Results
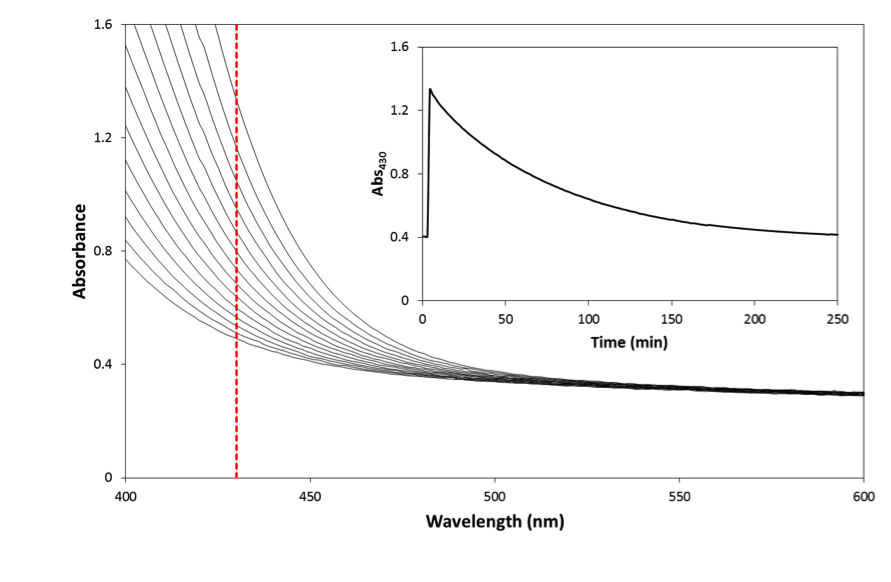
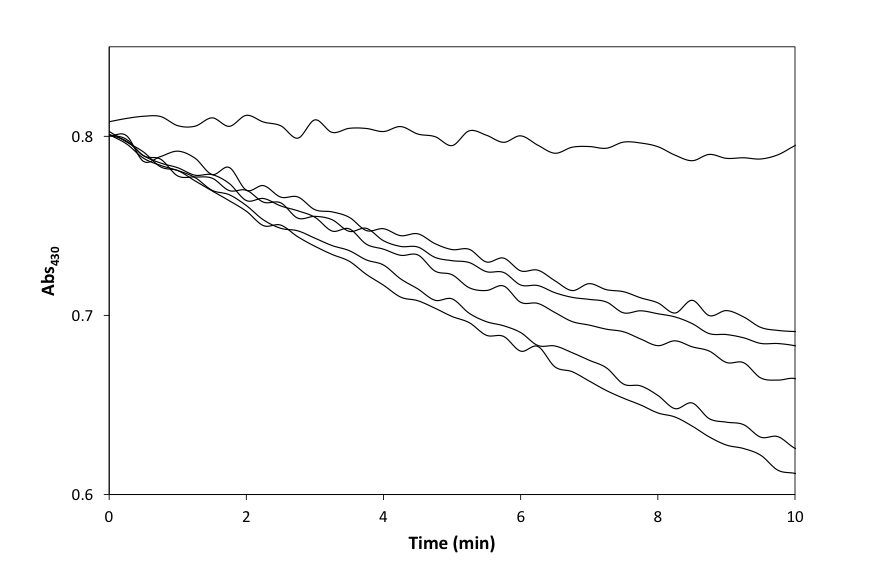
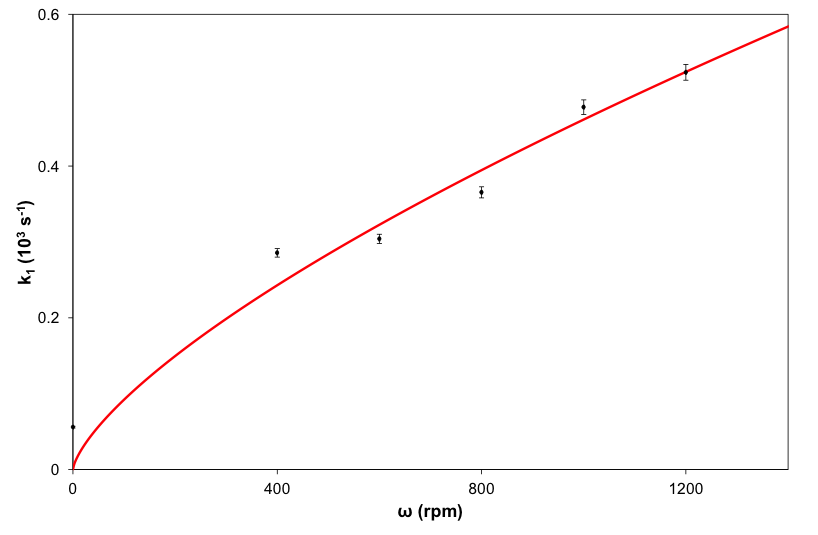
- Reaction (5) was studied initially using a dispersion of a ground up form of the heat-treated pencil lead and the results of this work are illustrated in Figure 2, which shows the change in the UV/Vis absorption spectrum of the 3.5x10-3 M Ce(IV) sulfate solution, in 0.5 M H2SO4 plus 2 M NaCl and 60 μg cm-3 pencil lead, as a function of time. Ce(IV) sulfate is yellow coloured with an absorbance maximum at 320 nm and molar absorptivity, ε(320) [20] of 5580 M-1cm-1 in 0.5 M H2SO4,. Thus, in this work, in order to monitor the decay kinetics of the Ce(IV) via its absorbance it was necessary to monitor its absorbance decay at a higher wavelength, where it does not absorb so strongly, which in this case was 430 nm (see Figure 2), since ε(430) = 235 M-1cm-1 in a solution comprising 0.5 M H2SO4 plus 2 M NaCl. A typical absorbance (at 430 nm) vs decay time profile, recorded using the above reaction conditions, is illustrated in the insert diagram in Figure 2. A first order analysis of the latter revealed an excellent straight line, with r2 = 0.999, over 3 half-lives and a value for k1 = 0.020 min-1 (i.e. 3.3x10-4 s-1). In a subsequent study of reaction (5), the initial decay of the Ce(IV), due to the redox catalytic activity of the rotated heat-treated pencil lead was also monitored spectrophotometrically at 430 nm, and as a function of rod rotation speed, in rpm, and the results of this work are illustrated in Figure 3. As noted earlier, in the rotating rod experiments, each run was monitored over the first 10 min, so that the whole experiment could be completed in 1.5-2 h; i.e. within an afternoon and so corresponds to the top part of the decay curve in Figure 2.
4. Discussion
- The high yield (79%) of chlorine produced when using the heat-treated pencil lead, in powder or rod form, to mediate reaction (5) indicates that this is the major reaction responsible for the reduction of Ce(IV). As noted earlier, the reduced yield with pencil lead graphite, compared to pure graphite, is probably because of adventitious impurities in the clay or remnants of the wax since the pencil lead is only ca. 60% graphite; cf. earlier results. The excellent first-order kinetics for reaction (5), observed using a dispersion of the ground-up, heat-treated, powder form of the pencil lead, as illustrated in Figure 2, suggests that Ce(III) does not interfere with the reaction and that the kinetics are most probably controlled by the rate of diffusion of the Ce(IV) ions to the graphite particles, in accordance with the electrochemical model of redox catalysis based on the likely current voltage curves for the two redox couples, illustrated in Figure 1. It is assumed that these same conditions hold when the heat-treated pencil lead is used as a rotating wire-less electrode.In the rotating pencil lead experiment, each of the decay curves, illustrated in Figure 3, gave a good fit to first order kinetics [r2 > 0.996] and a different value for k1 for each rotation speed, ω. This data was then used; to create the data plot shown in Figure 4. A subsequent plot of (k1/s-1) versus (ω/rpm)0.7, derived from eqn(11), yielded a good straight line of best fit [r2 = 0.996] with a gradient, m = (3.67 ± 0.18)x10-6, which then enabled the data for the solid line in Figure 4 to be generated using eqn (11), given m = 0.0315(h/cm)(D/cm2s-1)0.644(d/cm)1.4(υ/cm2s-1)-0.343/(V/cm3). The value of m = (3.67 ± 0.18)x10-6 was also used to calculate the following value for the diffusion coefficient for Ce(IV) in the 0.5 M H2SO4: D = (2.7 ±0.1)x10-6 cm2.s-1), given: h = 3.1 cm, d = 0.2 cm, υ = 0.01 cm2 s-1 and V = 3.5 cm3; this value compares well with those reported for Ce(IV) on Pt electrodes; i.e. (3.4-5)x10-6 cm2 s-1 [38, 39].
5. Conclusions
- A heat-treated rotating pencil lead can be used as a heterogeneous redox catalyst for the oxidation of chloride to chlorine by Ce(IV) ions in an aqueous solution comprising 0.5 M H2SO4 and 2 M NaCl. When used in ground-up powder form, the decay of the Ce(IV) is first order over 3 half-lives and the yield of chlorine is high, 79%. When used as a rotating 2 mm heat-treated pencil lead the first order rate constant, k1, for Ce(IV) reduction is found to depend directly upon ω0.7, where ω is the rod rotation speed. This latter finding is as expected based on the modelled hydrodynamics of the system and the assumption that the rate of Ce(IV) reduction depends upon the rate of diffusion of the Ce(IV) ions to the surface of the spinning pencil lead rod. The gradient of the k1 vs ω0.7 plot allows a value for the diffusion coefficient for Ce(IV) ions in 0.5 M H2SO4 plus 2 M NaCl solution to be calculated, namely: (2.7 ±0 .1)x10-6 cm2/s) which compares favourably with those reported previously by others [38, 39]. This simple set of experiments provides an introduction to, and novel illustration of, heterogeneous redox catalysis, which is a process that is common in industry, but usually not mentioned in undergraduate textbooks.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML