-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
p-ISSN: 2331-7450 e-ISSN: 2331-7469
2017; 5(3): 41-47
doi:10.5923/j.jlce.20170503.01

Enhancing Research Skills and Attitudes in Undergraduate Organic Chemistry with a Course-Embedded Undergraduate Research Experience (CURE) via Green Organic Synthesis
Patrick Coppock, Sang H. Park, Julia Paredes, Richard Pennington, David P. Pursell, Gillian Rudd, Joseph C. Sloop, Mai Yin Tsoi
School of Science and Technology, Georgia Gwinnett College, Lawrenceville, GA, USA
Correspondence to: Joseph C. Sloop, School of Science and Technology, Georgia Gwinnett College, Lawrenceville, GA, USA.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The Organic Chemistry II laboratory experience is reimagined as a semester-long green synthesis project involving the preparation of sulfa drugs. Student groups select a sulfa drug target to synthesize, research their assigned sulfa drug target, prepare a research plan and brief their instructor on their plan. Once their plan is approved, students prepare their experimental procedures before coming to lab, then synthesize, isolate, purify and analyze their sulfa drug product over the course of the semester. Through this project, students are exposed to a more research-focused laboratory experience. End-of-semester written synthesis exam scores indicate that students engaged in this project scored on average 8 percent higher on the synthesis exam than students who took the course prior to implementation of this project.
Keywords: Sulfonamide, Sulfa drug, Acetanilide,p-acetamidobenzenesulfonyl chloride
Cite this paper: Patrick Coppock, Sang H. Park, Julia Paredes, Richard Pennington, David P. Pursell, Gillian Rudd, Joseph C. Sloop, Mai Yin Tsoi, Enhancing Research Skills and Attitudes in Undergraduate Organic Chemistry with a Course-Embedded Undergraduate Research Experience (CURE) via Green Organic Synthesis, Journal of Laboratory Chemical Education, Vol. 5 No. 3, 2017, pp. 41-47. doi: 10.5923/j.jlce.20170503.01.
Article Outline
1. Introduction
- Chemistry faculty in the School of Science and Technology at Georgia Gwinnett College revised the second semester organic chemistry course laboratory program as part of a Science, Technology, Engineering and Math (STEM) II initiative [1]. Evidence clearly indicates that research experiences increase students’ interest in persisting in STEM programs leading to the STEM professions and Course-embedded Undergraduate Research Experiences (CUREs) are a popular method of providing these experiences [2-6]. The overarching goals were to (1) expose students to a research-like experience which embraced “green” chemistry principles and techniques using a multi-step organic chemistry synthesis of sulfonamides (aka “sulfa drugs”) at the miniscale level [7, 8], and (2) compare student performance on the synthesis exam before and after it had been introduced. See Scheme 1.
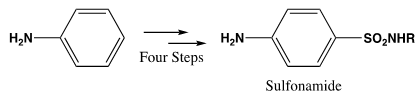 | Scheme 1. Sulfa Drug Synthesis |
|
2. Materials and Instrumentation
2.1. Materials
- a. Experimental Instructions (contained in the on-line Laboratory Text – Chemistry 2211K/2212K).b. Laboratory Technique Videos [11].c. Aniline, acetic anhydride, chlorosulfonic acid, 4-bromoaniline, 4-methylaniline, ethyl-4-aminobenzoate, 3,5-dimethylaniline, 4-acetylaniline, benzylamine, piperidine, N-methylpiperazine, 3-methylpiperidine, morpholine, 4-methoxyaniline, pyridine, acetonitrile, ethyl acetate, diethyl ether, hexanes and ethanol were purchased from Sigma-Aldrich Chemical company and were used as purchased.d. Magnesium sulfate, NaCl and HCl were purchased from Fisher Scientific Chemical company and were used as purchased.
2.2. Instrumentation
- a. Melting points were obtained on an SRS Digimelt MPA160 melting point apparatus and are uncorrected. b. IR spectra were collected on a Thermo Scientific Nicolet 155 FT IR spectrometer. c. 1H NMR spectra were collected on a Thermo Scientific PicoSpin-45 FT NMR operating at 44.90 MHz. [12]d. 13C NMR spectra were collected on an Anasazi-90 FT NMR operating at 22.50 MHz.
3. Experimental
- During the first laboratory session, instructors provide students an overview of the synthesis project, the pre-experimental activities which students must accomplish and a general synthesis reaction pathway (Scheme 2).
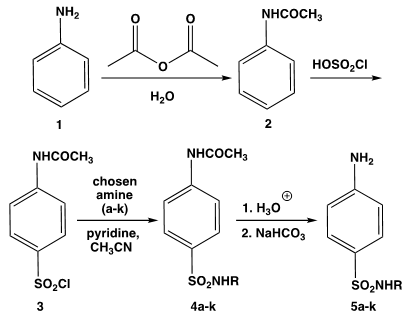 | Scheme 2. General Synthesis Pathway. |
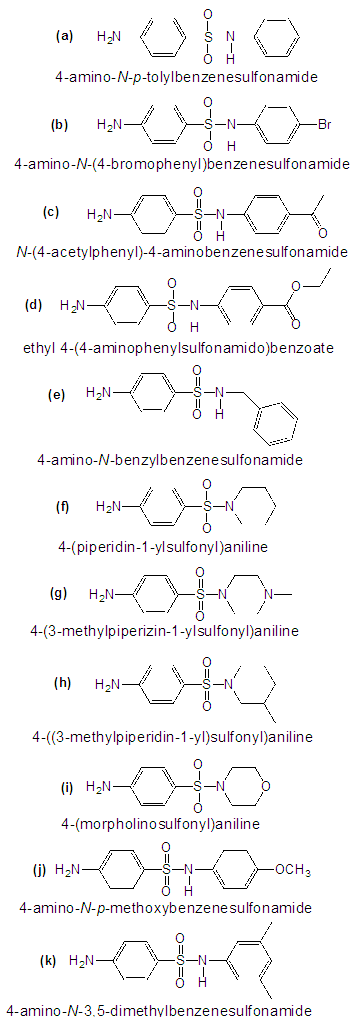 | Figure 1. Sulfonamide Targets |
3.1. Pre-Laboratory Activities
- Prior to conducting experimental work in the laboratory, students must complete several activities to prepare themselves for the project.
3.1.1. Literature Search
- Student teams first conduct a search of the scientific literature for synthetic preparations of their target sulfonamide. Teams are expected to retrieve and review at least two peer-reviewed journal articles related to sulfonamide synthesis. For many students, this leads to discovery of several published experimental procedures that offer synthesis pathway options they may consider [14]. Students discuss these options with their instructor to ascertain availability of reagents and materials. Instructors may elect to approve student team requests for special reagents, materials and procedures provided there is adequate time to acquire those items. Once student teams have identified viable articles, they prepare their synthesis plan briefing.
3.1.2. Synthesis Plan Briefing
- Although the exact format for the briefing is instructor-dependent, student teams will usually prepare a written presentation and present it orally to the instructor. Based on backward planning, the presentation will include a timeline which encompasses the entire project from beginning the synthesis operation in the laboratory through delivery of the final synthesis project report. Teams are expected to account for all aspects of the project. Teams present the synthesis plan detailing each reaction to be undertaken, including reactant quantities, mole ratios, reaction conditions (reaction mole table components), workup, isolation, purification as well as physical constant and spectroscopic characterization.
3.2. Laboratory Activities - General Preparative Procedures
- The laboratory manual contains a very general set of sulfonamide synthetic procedures. These are outlined below.
3.2.1. Synthesis of Acetanilide (2) [15]
- Chemicals used: aniline, acetic anhydride, distilled water. Equipment used: stir/hotplate, Erlenmeyer flask (size depends on scale that you wish to run the reaction), magnetic stir bar. For mole table: Use a 1:1.2 molar ratio of aniline:acetic anhydride. Procedure: To an Erlenmeyer flask equipped with a stir bar is added aniline. Distilled water (7.5 times the volume of aniline used) is then added to the flask with stirring. Acetic anhydride is then carefully added into mixture. A second aliquot of distilled water (2.5 times that of the original volume of distilled water used) is added to the mixture and the resultant mixture stirred with heating, until all solids and residual oils are dissolved. After dissolution, allow the solution to cool to room temperature, and then place in an ice bath to maximize crystal formation. Vacuum filter the crystals, washing with cold distilled water. Allow the crystals to dry completely (use the Fries lamp to dry the crystals more quickly). Calculate % yield. Determine m.p., and obtain IR and 1H NMR spectra of the reaction product, 2. Perform a TLC analysis by comparing your reaction product to the aniline starting material (use 3:1 ether:hexane as the elution solvent). Notes to Instructors: a. Student teams are provided 2.0 grams of aniline.b. Typical yields for this reaction exceed 70%.
3.2.2. Synthesis of p-Acetamidobenzenesulfonyl chloride (3) [16]
- Chemicals used: Acetanilide (from 1st step), chlorosulfonic acid, distilled water. Equipment used: Erlenmeyer flask, disposable glass pipettes, graduated cylinder (size depends on scale of reaction being run). For the mole table: Use a 5:1 ratio of chlorosulfonic acid:acetanilide. Safety Notes: 1. Chlorosulfonic acid is extremely hazardous – wear lab jackets, gloves and safety glasses when handling this chemical, and only do so inside a fume hood. You will note that when opened, the container emits fumes. Use extreme caution when dispensing this chemical, and only use glass pipettes to do so. 2. Chlorosulfonic acid reacts violently with water, and generating gaseous HCl in the process. Be aware of any sources of water close to your sample of chlorosulfonic acid. Procedure: In the hood, place dry acetanilide into a dry Erlenmeyer flask (flask size will be dictated by the scale of reaction). Very slowly and carefully, add the chlorosulfonic acid to the acetanilide (Safety Note 1). Try to avoid dripping any chlorosulfonic acid down the interior walls of the Erlenmeyer flask. After the chlorosulfonic acid has been completely added, place the Erlenmeyer flask into a warm water bath to complete acetanilide dissolution. After complete dissolution has been observed, allow the resultant oil to cool for several minutes. Once cooled, very slowly and carefully pipet portions of the oil into a beaker of ice-distilled water (Safety Note 2) until all oil has been added. Very carefully, run cold distilled water through the Erlenmeyer flask to react with any remaining oil, and add into the beaker of ice-water. The crude product, 3, will form a precipitate. Vacuum filter the product while washing it with cold distilled water. Vacuum dry to a moist solid consistency, and then dry further under the Fries lamp. Once the product is completely dry, calculate % yield. Determine m.p., and obtain IR and 1H NMR spectra of the reaction product, 3. If desired, perform a TLC analysis by the acetanilide vs the p-acetamidobenzenesulfonyl chloride, using a 3:1 mixture of ether:hexane as the elution solvent. The sulfonyl chloride product degrades under TLC conditions, so two spots may be observed for it. Note: p-acetamidobenzenesulfonyl chloride is hygroscopic, and so you will need to store your dry product in the desiccator (in a properly labeled vial) until the following week. Notes to Instructors: a. Encourage teams to be patient while executing this reaction. Student teams often have difficulty with dissolution of the reactant mix. If they attempt to begin transfer of the oil before dissolution is complete, a lower yield for the reaction results. b. During the subsequent oil transfer, placement of the pipette too close to the water-ice solution can result in solidification of the product in the pipette and thus impede product isolation. c. Yields for this reaction range widely from 30% - > 90%.
3.2.3. Synthesis of Acetylated Sulfonamide (4a-k) [17]
- Chemicals used: dried p-acetamidobenzenesulfonyl chloride from the previous step, selected amine (a-k), pyridine, acetonitrile. Equipment used: Erlenmeyer flask (or microscale reaction flask if reaction performed on a sufficiently small scale), stir/hot plate, magnetic stir bar. For the mole table: Use a 1:1.1 ratio of p-acetamidobenzenesulfonyl chloride: selected amine. Safety Note 3: Because pyridine is toxic and has a very strong, unpleasant odor, this entire procedure must be performed inside a fume hood. Procedure: In the hood, place the p-acetamidobenzenesulfonyl chloride into the reaction flask. To the flask add acetonitrile, using approximately 1 mL of acetonitrile per 100 mg of p-acetamidobenzenesulfonyl chloride (note that the p-acetamidobenzenesulfonyl chloride will not dissolve in acetonitrile). Dissolve the selected amine in pyridine (Safety Note 3), using approximately 1 mL of pyridine per 250 mg of amine. Add the pyridine-amine solution into the p-acetamidobenzenesulfonyl chloride-acetonitrile mixture, and stir at room temperature for several minutes (the reaction should take place quite rapidly). Check reaction progress by TLC (using 3:1 diethyl ether:hexanes as the elution solvent), stopping the reaction once TLC indicates complete disappearance of starting p-acetamidobenzene sulfonyl chloride. Acidify the solution with 2M HCl, and then extract with ethyl acetate. Wash the organic layer with (1) H2O and (2) saturated NaCl (aq), and dry it with anhydrous MgSO4. Concentrate the dry organic layer to retrieve the product, and recrystallize from an ethanol/water mixture (add a small amount of ethanol to the solid, and heat; then add a small amount of water to dissolve the solid). Dry the solid product under the Fries lamp.Once the product is completely dry, calculate % yield. Determine m.p., and obtain IR, 1H and 13C NMR spectra of the reaction product. Notes to Instructors: a. Encourage teams to be patient while executing this reaction. Student teams may have difficulty determining when the coupling reaction is complete by TLC. b. During the reaction work up, neutralization to remove the remaining pyridine and unreacted amine can pose problems if students do not correctly determine the pH of the aqueous layer during separation. In addition, insufficient organic layer washes will result in pyridinium and amine salt contamination of the acetylated sulfa drug. A TLC of the organic layer can quickly establish whether the organic layer is free from amine by-products.c. Yields for this reaction range widely from 20% - 70%. Amines containing electron-withdrawing groups typically give lower product yields for this reaction.d. The acetylated sulfa drug precursors have limited solubility in deuterated solvents, often requiring d6-DMSO to solubilize. This may require students to run multiple (more than 32) scans to acquire satisfactory 1H NMR spectra.
3.2.4. Synthesis of Sulfonamide (Sulfa Drug) (5a-k) [18]
- Chemicals used: acetylated sulfonamide (product from the previous step), 6M HCl, saturated NaHCO3 solution, pH paper. Equipment used: stir/hotplate, Erlenmeyer flask (or 10 mL round bottom flask and condenser), magnetic stir bar. Procedure: Place the acetylated sulfonamide (4a-k) into the Erlenmeyer flask, and add in 6M HCl (use approximately 5 mL HCl per 500 mg product). Stir the mixture and heat it to boiling (reflux if round bottom flask and condenser are used) until the solid dissolves. Cool the solution to room temperature, and add saturated sodium bicarbonate solution until a pH of 7-9 is obtained. Cool the mixture in ice to collect the precipitate the crude product and vacuum filter. Recrystallize the crude product (5a-k) from ethanol and dry using the Fries lamp. Once the product is completely dry, calculate % yield. Perform a TLC analysis by comparing your reaction product to the acetylated precursor (use 3:1 diethyl ether:hexane as the elution solvent). Determine m.p., and obtain IR, 1H and 13C spectra of the reaction product. Determine the overall % yield for the entire synthesis. Notes to Instructors: a. Encourage teams to be patient while executing this reaction. Student teams have difficulty determining when the deacetylation reaction is complete. b. During the reaction work up, neutralization to remove the acid can pose problems if students do not correctly determine the pH of the aqueous layer during separation. A TLC can quickly establish whether the product is free from any non-deprotected precursor.c. Yields for this reaction range widely from 20% - 70%. Recrystallization is often necessary to purify the final product.d. The target sulfa drugs have limited solubility in deuterated solvents, often requiring d6-DMSO to solubilize. This can require students to run multiple (more than 32) scans to acquire satisfactory 1H NMR spectra.
3.3. Post Laboratory Activities - Final Synthesis Report
- Instructors encourage teams to work throughout the semester on the report as the synthesis steps are completed. Following characterization of the final product, students prepare a research article in the Journal of Organic Chemistry format [19]. Students also orally present their project results to the instructor and/or classmates as part of their final report.
4. Synthesis Project Impact on Student Mastery of Organic Synthesis
- When this synthesis project was implemented as part of the STEM II initiative, several assessment metrics were used to determine its impact on student performance as well as student attitudes toward organic synthesis.
4.1. Synthesis Exam Mastery
- At the end of the second semester organic chemistry course at our institution, students are required to complete an open reference (without internet access), three-hour written Synthesis Exam. The exam emphasizes the thought processes involved in synthesis, including the scaffolding techniques introduced in the course and reinforced in the sulfa drug synthesis project, and requires students to think critically about how to best produce target molecules from given starting materials. This exam serves as a targeted assessment of student mastery of Course Outcome (CO) #2 (Table 1) [20].Prior to implementation of the sulfa drug synthesis project, assessments of CO #2 showed marginal student mastery of synthesis. After implementation, student mastery of CO #2 showed improvement. Table 2 compares the synthesis exam scores from before and after implementation of the synthesis project.
|
4.2. Student Attitudinal Surveys
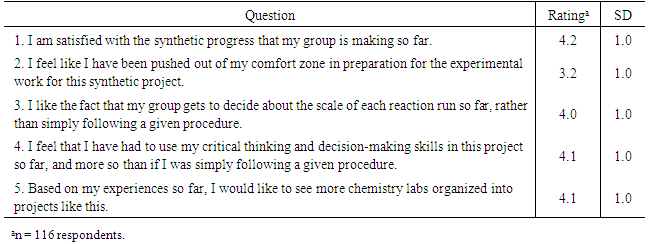
- One of the many factors of student achievement that have been previously studied is attitude towards science [21]. This term refers to “a general and enduring positive or negative feeling about science…” [22]. Studies conducted in the 1970s and 1980s indicated that this attitude could facilitate and produce cognitive learning in students, when present in conjunction with several other factors. A survey-type instrument was developed to measure student attitudes toward the sulfa drug synthesis project. Items were written to capture student attitudes about project progression, self-efficacy, independence, and personal development. A Likert scale was used in the survey to determine strength of student attitudes: 5=strongly agree, 4=agree, 3=neutral, 2=disagree and 1=strongly disagree. Table 3 summarizes the survey results.
|
|
5. Conclusions
- This marks the fifth year that this synthesis project has been in use at GGC. Course outcome assessment data suggests that student mastery of synthesis (as measured by the end of semester written synthesis exam) has improved since implementation of the sulfa drug synthesis project in 2012 by more than 8% compared to synthesis exam data prior to 2012. Overall, student surveys show while this semester-long project is challenging in terms of the preparation necessary and the uncertainty inherent in a research-like experience, the real-world applicability of drug synthesis, experience gained in experimental design and time management have made this project a valuable alternative to the usual slate of unrelated set piece experiments common to many undergraduate organic chemistry laboratory programs.
Supplemental Material
- A sample final report is available from the corresponding author upon request.
ACKNOWLEDGEMENTS
- The authors thank the Georgia Gwinnett College School of Science and Technology STEM II Initiative Grant for funding this work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML