-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
p-ISSN: 2331-7450 e-ISSN: 2331-7469
2017; 5(2): 26-31
doi:10.5923/j.jlce.20170502.03

Practical Activity for Development and Validation of a Simple UV-Spectroscopic Method for Iodate Determination in Table Salt
Rosa Garcia-Arrona, Mikel Arranz, Ane Bordagaray, Esmeralda Millán
Department of Applied Chemistry, Faculty of Chemistry, University of the Basque Country (UPV/EHU), Spain
Correspondence to: Rosa Garcia-Arrona, Department of Applied Chemistry, Faculty of Chemistry, University of the Basque Country (UPV/EHU), Spain.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

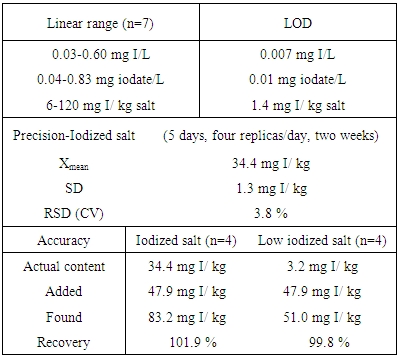
In this work a spectroscopic method with UV measurement for the iodate determination in salt was developed. The method is based on the reaction of iodate with iodide (in acidic medium) to form iodine and a buffered aqueous matrix was used. The analytical signal was measured at 352 nm using a simple spectrophotometer. The analytical curve for iodate was linear in the range of 0.04 – 0.83 mg/L (6 – 120 mg I/kg salt) with a correlation coefficient of 0.9999. The limit of detection and relative standard deviation were estimated at 0.01 mg iodate/L (1.4 mg I/ kg salt) and 3.8% respectively. The accuracy was assessed through recovery test (99.8 – 101.9%). Comparing this technique with the titration reference method in eight comercial table salts, no statistically significant difference was observed applying the paired t-test at 95% confidence level. The proposed UV method is a simple, economic and reliable alternative for the iodate determination in salt.
Keywords: Iodate determination, Method validation, UV spectroscopy, Table salt
Cite this paper: Rosa Garcia-Arrona, Mikel Arranz, Ane Bordagaray, Esmeralda Millán, Practical Activity for Development and Validation of a Simple UV-Spectroscopic Method for Iodate Determination in Table Salt, Journal of Laboratory Chemical Education, Vol. 5 No. 2, 2017, pp. 26-31. doi: 10.5923/j.jlce.20170502.03.
Article Outline
1. Introduction
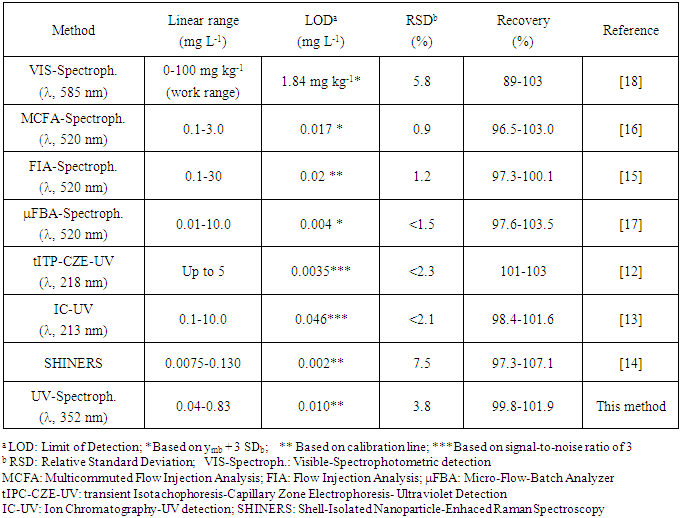
- Iodine is an essential element in human nutrition since it is a key component of the chemical structure of the thyroid hormones [1]. This element is important during the whole life, but especially in pregnancy, infancy and childhood. The iodine deficiency can lead to brain damage, mental retardation, neuromuscular defects and goiter [2]. WHO- UNICEF-ICDD recommend that the daily intake of iodine should be 90 g for preschoolers, 120 g for school children, 150 g for adolescents and adults and 250 g for pregnant and lactating women. About a third of the world’s population lives in areas with some iodine deficiency, being common in countries in the Eastern Mediterranean region, Asia, Africa and large parts of Easter Europe [3]. The iodized salt is recognized as the best strategy for the prevention and control of iodine deficiency disorders (IDD). To achieve the daily recommended level in adults, iodine in salt should be in the range of 20-40 mg/Kg [4]. Potassium iodate (KIO3) and potassium iodide (KI) are frequently used for salt fortification due to its higher iodine availability and low cost. The U.S. Food and Drug Administration (USFDA) recommend 60-100 mg KI/kg salt, equivalent to 46-76 mg I/kg salt [5]. WHO and UNICEF recommend potassium iodate since its greater stability, particularly in warm and humid conditions.Several analytical methods have been used for the iodide and iodate determination in salts. From the view point of simplicity and cost, iodometric redox titration is the chosen method [2, 6, 7]. Other methods using special instrumentation such us inductively coupled plasma mass spectrometry (ICP-MS) [5], ion chromatography (IC) coupled with ICP-MS [8], IC with amperometric detection [9] and high performance liquid chromatography (HPLC) with diode array detection [10] have been used. For iodate determination in table salt other researchers have proposed methods using potentiometry [11], transient isotachophoresis- capillary zone electrophoresis (tITP-CZE) [12], IC with ultraviolet (UV) detection [13] and shell-isolated nanoparticle-enhanced Raman spectroscopy (SHINERS) [14]. However, those analytical procedures involve high cost and require special equipment. Other options for the iodate determination are the spectrophotometric methods. A method with the hydroxylamine oxidation to nitrite followed by diazotization to produce the colored azo dye has been proposed [15]. Most of the procedures are based on the reaction of iodate with iodide to form molecular iodine. The formed iodine can react with the chromogenic N,N-diethyl-p-phenylenediamine [16, 17] or can form with the starch the blue iodine-starch complex that absorbs at 585 nm. This is the alternative for rapid test kits used for qualitative proposes and for hand-held instrumentation that allows quantitative measurements [11, 18]. However, it is possible to take advantage of the absorbance property in the UV region by the iodine formed in the reaction of iodate with iodide in acidic medium. The goal of the activity is to promote the interest of the students in the Analytical Chemistry trying to determine a nutritional analyte in real samples using simple, economical and reliable methods. We have taken as an example, the iodate determination in table salt using UV spectrophotometric determination. The work was done in the fourth course of the Bachelor’s degree in Chemistry. In the whole activity the considerations about the literature, the development of the procedure, the validation and the application to real samples were taken into account.
2. Experimental
2.1. Reagents and Solutions
- Analytical grade KIO3, KI, boric acid (H3BO3), sulfuric acid (H2SO4), sodium thiosulfate (Na2S2O3) and starch were purchased from Panreac (Barcelona, Spain). The solutions for iodate determination in salts using titration reference method were prepared as the indications of WHO-UNICEF-ICCIDD [2]. The solutions for spectroscopic method are shown in the next paragraph. The properly stored (cool, dark place, amber glass bottle) solutions are stable at least one month. Distilled water was used in all preparationsS1: Buffered KI solution. Dissolve 6.3 g of boric acid in 700 mL distilled water. Heat gently for boric dissolution and cool to room temperature. Add 10 g of KI and after dilution make to 1 L with distilled water. The shelf life of the buffered solution is approximately two weeks.S2: 0.1 M H2SO4. Carefully add 5.6 mL concentrated H2SO4 to 900 mL distilled water. Complete to 1000 mL.S3: 4.7 10-3 M KIO3. Dissolve 0.500 g de potassium iodate in 400 mL distilled water. Complete to 500 mL. S4: 4.7 10-5 M KIO3. Take 5.0 mL of S3 solution. Add 0.5 g KI and 10 mL of 0.1 M sulfuric acid (S2), dissolve and make to 500 mL with distilled water. Prepare just before the calibration solutions.
2.2. Samples
- Eight commercial table salts varying the total iodine (iodate and/or iodide) content were analyzed. Six of the samples showed in the labels total iodine contents around 6 mg per 100 g salt (60 mg I/Kg). However, there were not label specifications about iodate and/or iodide content.
2.3. Procedures
- The titration method for iodate determination in salt essentially consists of two analytical steps. In the first one, the free iodine is liberated from the salt; and in the second one, the free iodine is titrated with thiosulfate using starch as indicator. 50 g of iodated salts are thoroughly dissolved in 250 mL distilled water. From which an aliquot of 50 mL is analyzed as follows. Add 2 mL 1 M sulfuric acid solution and 5 mL of 10% potassium iodide solution. The iodine is then titrated with 2.5 10-3 M sodium thiosulfate solution using starch solution near the titration end point [2, 6]. In the proposed method the liberated iodine from the iodated in salt is determined using the absorbance measured at 352 nm and the calibration curve of absorbance vs. iodate (or I) concentration. The final solutions for UV measures are made in buffered solution (S1) in volumetric flasks. Due to the stability, this solution has been used in the determination of ozone in air at low concentrations [19, 20].Calibration solutions. Take 0.5, 1, 2, 3, 4, 5 and 10 mL from the S4 solution and complete to 100 mL with buffered solutions (S1) in volumetric flasks. The concentration of calibration solutions were from 0.04-0.83 mg iodate/L (0.03-0.60 mg I/L).Blank solution. Take 10 mL of of 0.1 M sulfuric acid (S2) and make to 100 mL with buffered iodate solution (S1) in volumetric flask. Sample solutions. Put 0.5 g of iodated salt in a beaker, add 10 mL of 0.1 M sulfuric acid (S2) and 50 mL buffered iodate solution (S1), transfer to a 100 mL volumetric flask and make to 100 mL with buffered solution.
2.4. Equipment
- Measurements were carried out using UVmini-1240 spectrophotometer (SHIMADZU, Kyoto, Japan) with a 1 cm quartz cuvette, at room temperature. Absorbance at 352 nm was measured in all the solutions.The UV spectrum was recorded by 8453 UV-Vis Agilent spectrophotometer (Agilent Technologies, Waldbronn, Germany).
3. Results and Discussion
3.1. Selection of Wavelength
- The absorption spectra from 200 to 400 nm of iodide, iodate and iodine were recorded. The iodate and excess iodide react to form iodine in acidic medium. The iodine and iodide have absorption maximum below 250 nm wavelength and iodate has not an absorption maximum in the range. For iodine there are two measurable absorption peaks found at 288 and 352 nm. The spectra of the three compounds are shown in Figure 1. Since the peak at 352 nm has been used to measure the iodine released from the reaction of ozone with iodide ions [19, 20], the analysis was carried out at 352 nm.
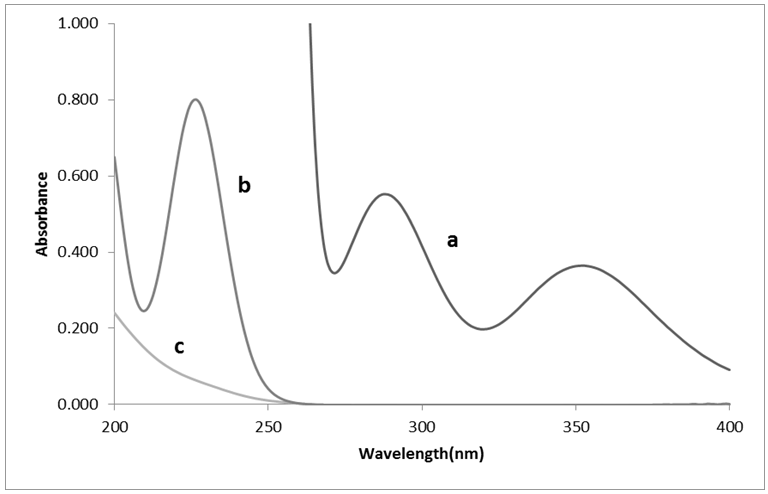 | Figure 1. Spectra obtained with independent solutions of: a) Iodine, b) Iodide and c) Iodate |
3.2. Analytical Characteristics
- In order to get the adequate solution for UV iodate determination preliminary experiments were done. With the aqueous solution the regression line obtained was not good enough (y= 0.1334 x – 0.018, R2 = 0.9803), and the obtained results in commercial salts gave higher values compared the ones of the titration procedure. In other works the problem was solved using phosphoric acid [21] or boric acid [19, 20]. Since the great stability offer by the latter, the solution with boric acid was chosen to follow the work.Four series of iodate standard solutions were prepared in buffered solution and measured at 352 nm. The calibration curves were obtained from the signals measured for seven different concentration levels (0.04-0.83 mg iodate/L; or 0.03-0.60 mg I/L). The obtained equation was y = 0.6073 x – 0.0034 (R2 = 0.9999); in which y is the absorbance and x is the iodide concentration. The linear range expressed in the usual units for table salts is from 6 to 120 mg I/kg. The averaged regression equation is shown in Figure 2.
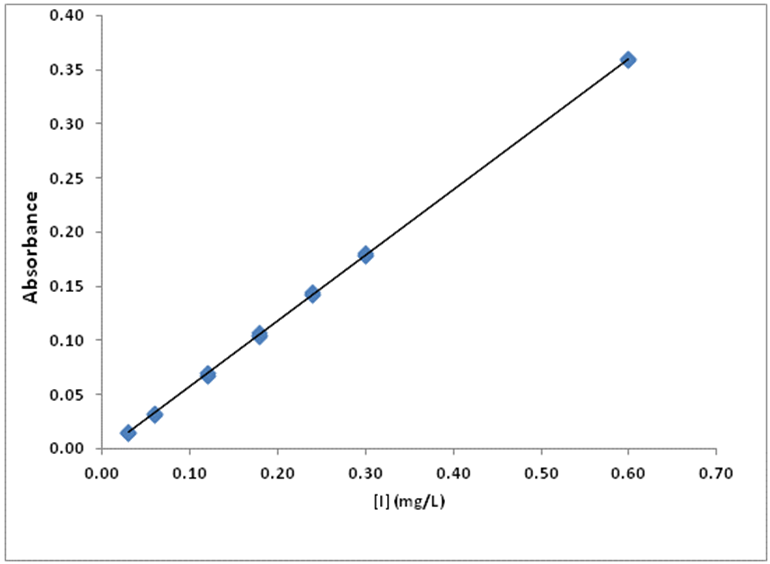 | Figure 2. Averaged calibration curve obtained with the UV measurements of the buffered standard solutions |
|
|
3.3. Application to Salt Samples
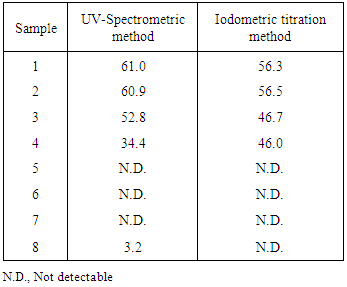
- In order to evaluate the applicability of UV-spectrometric method for iodate determination, eight comercial table salts were analyzed with UV-method and with the conventional iodometric titration. The results obtained for the iodate content with the two procedures, expressed as mg I/kg salt, are shown in Table 3. The application of paired t-test to the obtained data with UV-method and iodometric titration showed that there was no significant difference at 95% confidence level (texperimental=0.53, tcritical=2.36).
|
4. Conclusions
- In this work a reliable, low cost and simple method with UV measurement for determining iodate in salt has been developed. The procedure showed good accuracy and precision, and LOD obtained was comparable to other spectrophotometric methods. Also the comparison of the iodate content in table salts with titration conventional method showed no statistically significant differences. The requirements of sample size and reagents volume are small, and the proposed method avoids the use of chromogenic compounds or starch that are necessaries in visible spectrophotometric procedures. Another remarkable characteristic is the simple UV-Vis equipment used, that can be also considered as portable instrumentation, and allows the acquisition by laboratories with reduced financial support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML