-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
p-ISSN: 2331-7450 e-ISSN: 2331-7469
2017; 5(1): 6-8
doi:10.5923/j.jlce.20170501.02

Balancing Redox Chemical Equations: A Discovery Procedure Employing Oxidation Reduction Titration
S. Ghaffari, P. K. Thamburaj, S. Abu-Baker, Annette Holstein
Chemistry Department, Ohio University Zanesville, Zanesville Ohio, United States
Correspondence to: S. Ghaffari, Chemistry Department, Ohio University Zanesville, Zanesville Ohio, United States.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The relationship between an oxidizing agent and reducing agent may be established by a volumetric procedure known as redox titration. Potassium permanganate, KMnO4, is a favorite oxidant partly because of its color which serves as the indicator. Iron (II) ion, Fe2+, as reducing agent is titrated with KMnO4 to determine the oxidation state of Mn in KMnO4. In the second titration oxidation Oxalate ion, C2O4-2, is used as a reducing agent against the KMnO4. Data obtained from titrations will lead to balancing each redox chemical reaction in an acidic medium.
Keywords: Balancing Chemical Equation, Oxidation/Reduction, Titration/Volumetric Analysis, First-Year Undergraduate/General Chemistry
Cite this paper: S. Ghaffari, P. K. Thamburaj, S. Abu-Baker, Annette Holstein, Balancing Redox Chemical Equations: A Discovery Procedure Employing Oxidation Reduction Titration, Journal of Laboratory Chemical Education, Vol. 5 No. 1, 2017, pp. 6-8. doi: 10.5923/j.jlce.20170501.02.
1. Introduction
- A chemical equation is a symbolic representation of a chemical reaction. To satisfy the law of conservation of mass (matter) these equations must be balanced. Balanced chemical equations are essential to solve problems in stoichiometry.In essence, balancing chemical equations is a mathematical procedure. Most chemical equations may be balanced by a simple trial and error or inspection technique. However, for chemical reactions labeled as redox reactions, it may seem that there is no simple method for balancing. There is a large number of articles published dealing with a variety of redox reactions [1-13]. These range from inspection to algebraic method.In redox equations, the number of electrons transferred from a reducing agent (oxidized substance) to an oxidizing agent (reduced substance) has to be balanced as well. In general, a redox equation can be expressed as
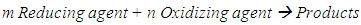 | (1) |
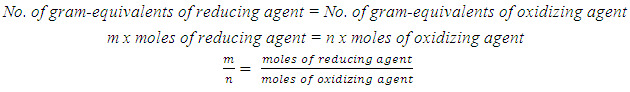 | (2) |
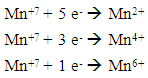 To find the ratio of “m/n”, molarity and the volume of reducing agent solution used in titration and molarity of MnO4- solution are provided. The volume of MnO4- required to reach the equivalence point is obtained by a titration experiment.Using a redox titration approach to find electron change of the oxidizing agent, KMnO4, and balancing redox equations involving KMnO4 at an introductory chemistry level is described here.
To find the ratio of “m/n”, molarity and the volume of reducing agent solution used in titration and molarity of MnO4- solution are provided. The volume of MnO4- required to reach the equivalence point is obtained by a titration experiment.Using a redox titration approach to find electron change of the oxidizing agent, KMnO4, and balancing redox equations involving KMnO4 at an introductory chemistry level is described here.2. Methodology
- Part I.
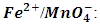 The oxidation reduction reaction of Fe2+ with KMnO4 is presented by an unbalanced equation with “m” and “n” as coefficients of
The oxidation reduction reaction of Fe2+ with KMnO4 is presented by an unbalanced equation with “m” and “n” as coefficients of 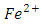 ion and
ion and 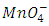 ion. These coefficients balance the number of electrons transferred from a reducing agent to an oxidizing agent.
ion. These coefficients balance the number of electrons transferred from a reducing agent to an oxidizing agent.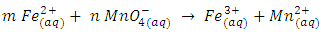 | (3) |
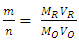 | (4) |
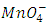 for the oxidizing agent.
for the oxidizing agent. 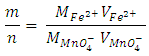 The method of finding the ratio of “m/n” molarity and the volume of Fe2+ solution and molarity of MnO4- solution are provided. The volume of MnO4- required to reach the equivalence point is obtained by titration.Student-generated experimental results give
The method of finding the ratio of “m/n” molarity and the volume of Fe2+ solution and molarity of MnO4- solution are provided. The volume of MnO4- required to reach the equivalence point is obtained by titration.Student-generated experimental results give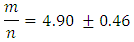 This will be presented as the ratio of two whole numbers
This will be presented as the ratio of two whole numbers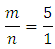 “m” and “n” in Eqn 3 are replaced by their values “5’ and “1” respectively and coefficients of the product side are added accordingly to balance elements that are oxidized “Fe” and reduced “Mn”.
“m” and “n” in Eqn 3 are replaced by their values “5’ and “1” respectively and coefficients of the product side are added accordingly to balance elements that are oxidized “Fe” and reduced “Mn”.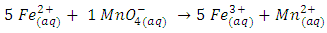 Iron has only two oxidation states “+2” and “+3” and there are five electrons exchanged between the reducing agent and the oxidizing agent. Therefore, Mn in KMnO4 must have an oxidation state of “+7”.To complete the balancing of the reaction, oxygen and hydrogen must be balanced, too. To balance the number of oxygens for each oxygen needed, one molecule of H2O is added. Since this reaction is in acidic medium, to balance hydrogen H+’s are added to complete the balancing of the redox reaction.
Iron has only two oxidation states “+2” and “+3” and there are five electrons exchanged between the reducing agent and the oxidizing agent. Therefore, Mn in KMnO4 must have an oxidation state of “+7”.To complete the balancing of the reaction, oxygen and hydrogen must be balanced, too. To balance the number of oxygens for each oxygen needed, one molecule of H2O is added. Since this reaction is in acidic medium, to balance hydrogen H+’s are added to complete the balancing of the redox reaction.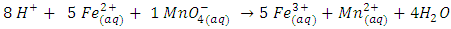 Part II.
Part II. 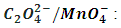 In the second titration oxalate ion,
In the second titration oxalate ion, 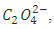 is used as the reducing agent in titration with KMnO4.
is used as the reducing agent in titration with KMnO4.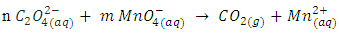 | (5) |
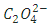 is a substitute for a reducing agent and
is a substitute for a reducing agent and 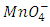 for the oxidizing agent.
for the oxidizing agent. 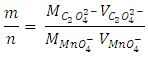 Student-generated experimental results give
Student-generated experimental results give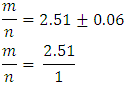 To round this ratio to a ratio of two whole numbers gives
To round this ratio to a ratio of two whole numbers gives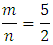 “m” and “n” in Eqn 5 are replaced by their values “5’ and “2” respectively and coefficients of the product side are added accordingly.
“m” and “n” in Eqn 5 are replaced by their values “5’ and “2” respectively and coefficients of the product side are added accordingly.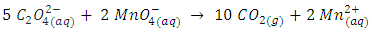 To complete balancing the reaction in an acidic medium, oxygen and hydrogen must be balanced following the procedure described previously. The final balanced equation is,
To complete balancing the reaction in an acidic medium, oxygen and hydrogen must be balanced following the procedure described previously. The final balanced equation is,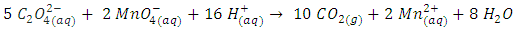
3. Conclusions
- This method is student friendly and provides hands-on experience and confirmation of the algebraic method of balancing redox reactions. Further study is needed regarding the application of this method in organic redox reactions and the use of other oxidizing agents such as dichromate.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML