-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
p-ISSN: 2331-7450 e-ISSN: 2331-7469
2017; 5(1): 1-5
doi:10.5923/j.jlce.20170501.01

Mystery Lab for Organic Chemistry
Jason C. Thoen, Makenzie Grover
College of Health and Wellness, Northwestern Health Sciences University, Bloomington, MN, USA
Correspondence to: Jason C. Thoen, College of Health and Wellness, Northwestern Health Sciences University, Bloomington, MN, USA.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The “Mystery Lab” is a laboratory experience that allows students to apply critical thinking, writing, and organic chemical laboratory skills. Students are challenged to solve a medical mystery presented as a short story that includes both situational information as well as clues to needed procedures. The students must identify which of a number of samples is tainted with a poison, separate the poison from the rest of the sample, then identify the poison using provided analytical data. Students complete the lab report by writing the end of the story presented in the lab handout. Creativity is encouraged!
Keywords: Critical thinking, Organic laboratory skills, Writing
Cite this paper: Jason C. Thoen, Makenzie Grover, Mystery Lab for Organic Chemistry, Journal of Laboratory Chemical Education, Vol. 5 No. 1, 2017, pp. 1-5. doi: 10.5923/j.jlce.20170501.01.
Article Outline
1. Introduction
- First term Organic Chemistry laboratory experiments often focus on development of laboratory skills that can be applied toward purification and/or analysis of organic compounds. While this is important and quite necessary, it does not automatically foster development of higher-order skills as defined by Bloom’s Taxonomy [1]. It also can be challenging for these experiments to prove engaging to the typical chemistry student. In an attempt to address these issues at our own institution, we have developed an experiment that gives students a chance to solve a mystery presented in a story format through application of organic lab techniques, and create their own ending to the narrative. Note: the population of students is composed almost exclusively of pre-health (medical, PA, PT, chiropractic, nursing, etc.) students.
2. The Story
2.1. Plot Summary
- The lab handout primarily consists of a short story following the experiences of David, a new intern in an analysis lab. Left alone in the lab while everyone else attends a conference, he is presented with a challenge that seems impossible to solve without use of the equipment for which he has not yet been trained. Someone has been poisoned, and David can’t analyze the biological samples yet. In order to determine the identity of the poison right away, David falls back on his experiences in organic chemistry labs to help him analyze samples found in the patient’s home.Samples of vanilla, cinnamon, mint, and almond extracts found in the patient’s home are analysed by David. He identifies the sample containing the poison, then separates the poison from the extract using basic organic laboratory techniques.David is ultimately successful in determining the poison after comparison of IR and 1H NMR spectra of the purified poison to that of the authentic chemical. He not only manages to solve the mystery before everyone comes back to the lab, but also gains a new appreciation for how much he learned in organic chemistry lab!The story is written without revealing the identity of the poisoned sample or the specific poison used. Hints are given in the story to help guide students to choose the most productive techniques that will lead to solving the mystery.
2.2. The Story
- The second week of David’s internship in the laboratory started even more slowly than he could have imagined. While most of his fellow postbac premed students landed scribe positions or work in medical research, all he could get was work in what amounted to little more than a (gasp) chemistry lab affiliated with the hospital. Even worse, the lab was completely deserted, with all of the scientists gone to a conference for most of the week. “It looks like you will have a quiet second week” said Tamara, his annoyingly cheery boss. Her parting words as she left were: “Read through the rest of the instrumentation manuals and we can start your training on the instruments at the end of the week. Then the real fun can start!”The real fun had certainly not started yet. Although he was a whiz with the IR after using it in orgo lab, he had never directly touched an NMR, GCMS, LCMS, or the one that sounds like it got its name from Ramen noodles. “I don’t even know what most of these things do”, David thought. “How am I going to do anything useful here and get a good rec letter? Or at least do something that I can bear to tell everyone about at lunch after they brag about their medical experiences?”Just when his self-pity was threatening to surpass his boredom level, he noticed that the box for incoming analysis requests was no longer empty. A thin Manila folder sat underneath a wide plastic jar with several small bottles and jars in it. As he was about to pick up the file and take a look, the phone started ringing. He got it on the third ring and blurted out “Hello”, then added, after a pause, the rest of what he had been told to say….. “Analysis lab four.” A commanding and impatient voice spat out: “This is Dr. Myers. I need to speak to Tamara. We have an interesting case that needs her immediate attention. A man may have been poisoned, and our efforts to analyze his blood for toxins have failed” Dr. Myers was not thrilled with the news that a newbie was alone in the lab until late in the week. “We need to get some answers on this case, and if your lab isn’t prepared to figure this out ASAP, we will find one that is!” “Swell”, David thought after the tell-tale click of a hang-up. “Six days on the job is all it took for me to get into hot water.” Lacking a specific plan, he went back to the file that was causing all of the commotion. On the top of the file was the hospital admission chart. There were no real surprises, except that the patient information gave the appearance of normally a perfectly healthy individual. Age: 33Gender: MaleKnown allergies: NoneCurrent Medications: NoneChief Complaint: HeadacheHistory of Present Illness:“John Smith is a 33 year old male who presents to the emergency department with a headache. The patient reports experiencing a severe headache that started two hours after a regular breakfast at approximately 1100 hours this morning. He states his breakfast consisted of coffee and toast. He notes taking 3 pills of an unknown analgesic in attempt to alleviate his pain, which offered no relief. The patient also reports experiencing a sudden onset of bleeding gums, yellow skin, and loss of energy, which developed over the past hour. He currently rates his pain a 10/10 and reports his headache is constant and increasing in intensity. Patient denies nausea, vomiting, chills, fever, cold, cough, chest pain, or any other flu-like symptoms. He also denies experiencing any abdominal pain, constipation, diarrhea, dysuria, hematuria, or any other related symptoms. The patient denies any other additional or worsening symptoms other than those described above. No further concerns or complaints have been voiced.”He reviewed the symptoms again before continuing to read the rest of the chart: headache, a sudden onset of bleeding gums, yellowish skin, and loss of energy. The diagnosis, agreed upon by several physicians, was that the symptoms were all caused by damage to the liver. But what was the reason for liver damage? That was the question his lab was supposed to answer. But what was he supposed to do? The analysis lab was clearly not firing on all cylinders, but something had to be done. The way he saw it, he had two choices: call Tamara now and whine that someone called and got mad that she wasn’t there, or try to figure things out himself and call her later with an answer to the problem. Not one to go running off for help without trying himself, he decided to look at the rest of the folder. Following the patient's chart was a lab analysis on the over the counter medications from the patient's home, that was performed shortly after the patient’s admission. The results concluded that the medicines from the patient’s home were pure and not contaminated. There was one sheet in the back of the envelope, which was a blurb scribbled at the bottom of the page, looking like it was a last minute discovery or an afterthought that was not expected to matter:“Coffee was home-brewed and flavored by patient using his own extracts to make what he called ‘mint almond vanilla delight’. The coffee grounds were from a mostly empty container that the patient had used many times previously without incident. The flavorings and analgesic samples all appeared quite new.”At this point, there was little else to do except label the samples and enter the information into the database (one of the few things he felt qualified to do at this point.) He pulled out the bottles one by one, copying down the information from each label and making his own list:1. Almond extract2. Vanilla extract3. Mint extract4. Cinnamon oilIt was extremely frustrating to have the samples that may hold the answer to the mystery in his hands and be unable to do anything about it. If he knew what was causing the problem, he could get an IR and could probably beg someone from another lab to run an NMR. But so what? He did not know which sample might contain the problem, or have a way to get the offending poison separated from the rest of the solution. “This stinks! I am completely useless! There is nothing I can do!”Or is there? What about the techniques from orgo lab? Could those be used to figure this thing out? He decided to give it a try. After a Google search of the molecules in the samples and a quick trip to the grocery store, David started his low-tech analysis of the samples included with the file. By the time he finished working through lunch, David’s cry of victory rang through the empty lab: “Yeeessssss! Nailed it!” After sheepishly looking around to make sure nobody had walked in to see his awkward attempts at a victory dance, David came back down to reality. “OK, I know which sample has an extra chemical in it. That’s a great start, but I have no clue what it is. And…. if this is all I can do on my own then I will have to give this to someone to finish. I can guess who won’t get the credit if that happens.”Frustrated, and with little else he could do, David went online and started looking for information on the sample that was tainted, and found a possible lead. The toxin he found was fairly nonpolar. “Hmmm”, thought David, “This molecule is less polar than the one it is mixed with, can I take advantage of it somehow?” After staring blankly at his notes for a full ten minutes, he tried a different tactic. “What methods do I know that can separate organic chemicals? Which ones could work best here?” After going over the list of options and looking up the physical properties of the two compounds, David started to get an idea. “So what I basically need to do is separate the known chemical from the unknown,” he said. But then he paused and said, “Wait a minute. I basically just said the answer to my problem! Could it really be this simple and basic of an answer?”Late Monday afternoon, David called Tamara’s cell and had the following conversation:“That’s right Tamara. Dr. Myers was determined to find out what made his patient sick, and fast. He was pretty rude on the phone, and actually sent someone this afternoon to take back the file and the samples from our lab. I enjoyed sending him back with the answer instead, and IR and NMR spectra for evidence. Let me tell you how I did it….”
3. Materials
- Ÿ Thin Layer Chromatography plates, silica gel, 254 nm fluorescent indicator. Ÿ UV lamp, 254 nm Ÿ EthanolŸ CoumarinŸ VanillinŸ Authentic samples: extracts purchased from grocery store, 1 mL extract diluted to 5mL (except mint, 2 mL diluted to 5 mL) with ethanolŸ Patient samples: extracts prepared as above but with addition of 10 mg coumarin to patient vanilla sampleŸ Diethyl etherŸ 1 M Sodium HydroxideŸ Sodium SulfateŸ Ethyl AcetateŸ Petroleum EtherŸ Standard organic laboratory equipment/glasswareŸ Disposable nitrile gloves
4. Experimental
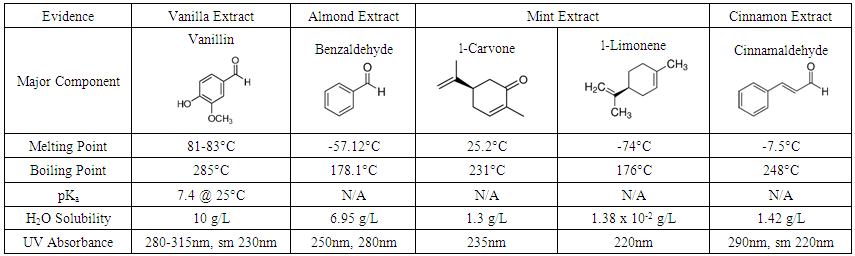
- Students are not provided with a procedure to follow. They are given the short story and a data sheet (Table 1) containing structural information and properties for the major components of the flavorings listed in the story.
 | Table 1. Sample Data Sheet |
 | Figure 1. Diagram of Experimental Procedure |
4.1. Prelab
- Students begin the lab with discussion of written initial plans for analysis with the lab instructor or assistant. Approval of students’ plans is a prerequisite for starting the experiment. Students are advised to use the provided disposable gloves to prevent direct skin contact with the poison as well as to use standard safety precautions.
4.2. Identify Sample with Poison
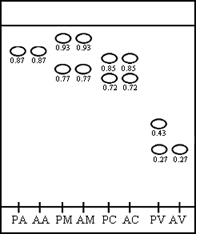
- Students have to decide on a method to determine which sample contains the poison. Thin Layer Chromatography is best suited for analysis of the samples (pairs of samples from the patient and authentic versions of each sample) all of which with major components that can be visualized using their UV absorbance at 254 nm. Upon deciding on TLC, students are provided with developing solution (20% ethyl acetate: petroleum ether). A picture of a typical TLC is shown in Figure 2; typical Rf values for each sample are detailed in Table 2. The TLC results reveal vanilla extract as the sample containing poison.
4.3. Separate Poison from Main Component of Sample
- In order to progress through the experiment, students must present a plan for purification of a mixture of the contaminated vanillin and the poison. Hints are given in the lab handout story: “The poison is less polar than the one it is mixed with, can I take advantage of it somehow?”…“So what I basically need to do is separate the known chemical from the unknown,” he said. But then he paused and said, “Wait a minute. I basically just said the answer to my problem! Could it really be this simple and basic of an answer?” If hints are required, students are directed to these passages to help them decide on acid/base extraction to separate vanillin (pKa of 7.4 for phenolic –OH) from what is described as a nonpolar poison. Dissolution of a provided 0.5 g 50:50 mixture of poison and vanillin in ether, followed by microscale extraction with 1 M NaOH results in a pure solution of the poison in ether. Transfer of the ether layer to an Erlenmeyer flask, drying with Na2SO4, decanting and evaporation of the solution results in a purified sample of the poison.
4.4. Confirm Separation Success
- Students can confirm successful separation of the poison from vanillin by showing that a TLC of the sample shows the spot at an Rf value of 0.43 but lacks the lower spot at Rf value of 0.27.
4.5. Obtain Data
- Students obtain a melting point on the purified solid poison and are then provided with IR and NMR spectra. Alternatively, students could obtain their own spectroscopic data, time permitting, time permitting. As the length of the experiment can be variable, we have simply provided spectra to students to ensure all can finish during the laboratory period.
4.6. Determine Identity of Poison
- Students are encouraged at this point to search for information regarding likely poisons that may be found as contaminants in vanilla extract. Many different sources cite the presence of coumarin in low quality vanilla extracts, especially those originating from Mexico [2]. Comparison of 1H NMR and IR data for matching peaks, as well as obtaining an experimental melting point close to the published value of 71°C, confirms this as the identity of the poison.
4.7. Exit Survey
- Upon completion of the experiment students are asked to fill out a short survey.
5. Results and Discussion
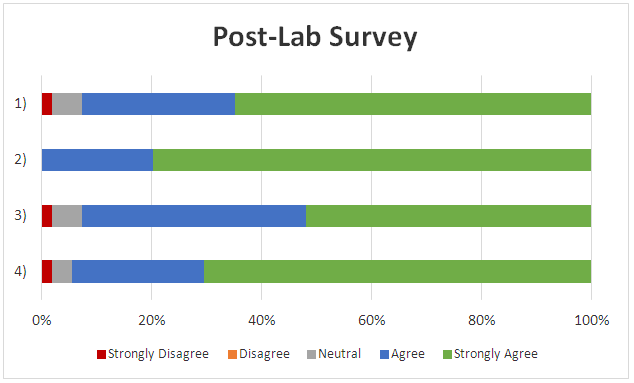
- Students are required to detail their experimental procedure and explain their results in the laboratory report. Additionally, students complete the story from the lab handout, inventing a specific explanation for the origins of the poisoned sample. Student submissions range from straightforward (subject bought vanilla in Mexico) to elaborate (revenge plots, the butler did it, etc.).The results of the exit survey, (turned in by 53 students over the course of three semesters) shown in Table 2 below, detail the students’ perceptions of the laboratory experience.Based on the survey data, students feel the experiment did succeed in challenging their critical thinking skills (100% positive response). Students also strongly believed that this experiment provided confirmation of their personal development of laboratory skills (93% positive) and managed to provide a positive learning experience (93% positive) that should be provided to future students (94% positive).Future developments for this experiment will focus on variations of the sample containing the poison and expansion to include different purification and/or analysis techniques.
6. Safety and Waste
- Students are required to use standard safety precautions, including the use of approved eye protection and gloves. The poison used in this laboratory exhibits oral LD50 values between 200-300 mg/kg, but is described only as an irritant in the event of skin and eye contact [3]. Students are provided samples containing less than a total of 500 mg of coumarin. All organic waste generated in this experiment can be combined in a general non-halogenated liquid organic waste container.
7. Conclusions
- The experiment described in this paper provides students with an opportunity to demonstrate competence in organic chemistry laboratory techniques and develop their critical thinking skills. Students have provided very positive feedback toward this experiment and many have expressed increased confidence in their ability to solve problems and apply their knowledge to new challenges.
ACKNOWLEDGEMENTS
- The authors wish to thank Brian Navoa, Frank Farleo, and Jakob Weber for submitting to a trial run of the experiment and for providing valuable insights from students’ perspectives.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML