-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
p-ISSN: 2331-7450 e-ISSN: 2331-7469
2016; 4(3): 45-52
doi:10.5923/j.jlce.20160403.01

Solvatochromism: A Comprehensive Project for the Final Year Undergraduate Chemistry Laboratory
D. González-Arjona1, G. López-Pérez1, M. M. Domínguez1, A. G. González2
1Department of Physical Chemistry (Faculty of Chemistry), University of Sevilla, Sevilla, España
2Department of Analytical Chemistry (Faculty of Chemistry), University of Sevilla, Sevilla, España
Correspondence to: D. González-Arjona, Department of Physical Chemistry (Faculty of Chemistry), University of Sevilla, Sevilla, España.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

A project for last year students of Chemistry based on solute-solvent interactions from the Physical Chemistry point of view is presented. The UV-Visible solvatochromic effect is used to characterize the solvent polarity. Three different solvatochromic probes, 4-Nitroanisol, 4-Nitrophenol and Reichardt’s dye Betaine (ET30) have been used to estimate the polarity parameters for eight organic solvents. Kamlet-Taft and Dimroth–Reichardt solvent scales are utilized to estimate the solvent polarity via parameters related with the dispersive forces, dipolar interactions and the tendency to H bond formation. Inside the framework of Linear Free Energy Relationships (LFER), literature data have been utilized to check the validity of these solvent models. The pKa values are analyzed to show the influence of the aprotic solvent polarity in the chemical equilibrium. The change in chemical rate constant of solvolysis due to the solvent polarity displays their possible influence in reaction mechanism. Good replications are obtained with the solvatochromic parameters by means of multi-linear regression relationship, for both LFER data.
Keywords: Solvatochromism, Solvents properties, Solvent scales, Betaine ET30
Cite this paper: D. González-Arjona, G. López-Pérez, M. M. Domínguez, A. G. González, Solvatochromism: A Comprehensive Project for the Final Year Undergraduate Chemistry Laboratory, Journal of Laboratory Chemical Education, Vol. 4 No. 3, 2016, pp. 45-52. doi: 10.5923/j.jlce.20160403.01.
Article Outline
1. Introduction
- Solvents are chemicals with a fundamental importance. They produce the dissolution and disintegration of one or more other substances obtaining a single homogeneous phase in the dissolution process. They have an undeniable importance in different areas of chemistry. Thus, chemical reactions performed in solubilized media usually take place faster and more easily. In addition, the choice of the right solvent in a chemical reaction is fundamental for an improved specificity and selectivity of reaction products.From the Thermodynamic point of view, two types of contributions are involved in the dissolution process. The first one is the purely entropy-driven mixing process, usually favored if an entropy increase is obtained simply as result of increasing the number of possible arrangements of molecules. The second one comes from enthalpic considerations, mainly derived from the interactions between the solvent and the substance to be dissolved (the solute), and the interactions of the solute and of the solvent among themselves. If the solute-solvent interactions are very strong (attractive), the solution process is certainly favored. On the other hand, from a kinetic point of view, a fast dissolution process can be expected if the solute is already a liquid, provided that the process is favored from the thermodynamic point of view.There is a rule of thumb in terms of the solubilization, "simila similibus solventur" or “like dissolves like”. This is an indication that the type of interactions between solvent and solute particles prior to mixing should be similar for the formation of a solution to be favored. The solvent characteristics are marked by kind of its interactions. A solvent classification can be useful to optimize their use.Solvents are generally divided into two large groups: polar and non-polar. The physical parameter associated with this classification is the relative dielectric permittivity (ε). Solvents with values of 15 or higher are considered polar. If additional considerations are taken into account, such as the formation of hydrogen bonds, polar solvents are classified as protic and aprotic.This classification scheme only provides a qualitative characterization of solvents. As usual, the boundaries between the different categories are not sharp. It would be interesting to have a scale that provides quantitative information on the specific nature of a given solvent. In particular, it would be desirable that this scale is based on physical properties that can be easily determined experimentally, such as relative dielectric permittivity, dipole moment, specific gravity, boiling point, etc. It is expected that the combination of these physical parameters will provide a more refined solvent polarity scale.Thinking along these lines, Hilderbrand’s solubility scale, that uses a single parameter, cohesive energy density, was one of the first of this kind to have been proposed [1]. More elaborate parametric scales have been developed: Hansen’s solubility scale uses three parameters: δD, taking into account the dispersion forces, δP the bonding polarity and δH the hydrogen bonds [2].
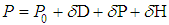 | (1) |
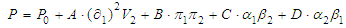 | (2) |
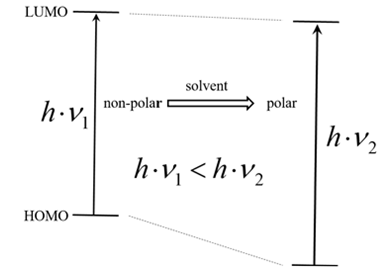 | Figure 1. Schematic energy scheme for a solute exhibiting a hypsochromic shift in solvents of increasing polarity |
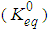 , is linked to the standard reaction free energy change
, is linked to the standard reaction free energy change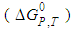 at constant T and P, by the familiar equation:
at constant T and P, by the familiar equation: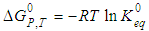 | (3) |
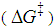 , can be assigned. Consequently,
, can be assigned. Consequently, 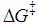 should also be affected by the nature of the environment.Solvent selection, in the realm of organic synthesis, is a fundamental task. The choice of solvent can greatly influence the type, isomerism and yield of the compounds obtained. Among the possible types of organic reactions, nucleophilic substitution is very common and useful [21]. These reactions are classified as SN1 and SN2 depending on the reaction order of the rate-determining step, unimolecular and bimolecular, respectively. The mechanism for SN1 is associated with the formation of a carbocation as the rate-determining step. The SN2 mechanism is postulated as bimolecular where the transition state is electrically neutral. Therefore, the solvent effect observed for a substitution reaction can help to elucidate their mechanism [22].Figure 2 displays simplified schematics of transition states for SN1 and SN2 which permits a qualitative prediction of rate constants from the relative values of
should also be affected by the nature of the environment.Solvent selection, in the realm of organic synthesis, is a fundamental task. The choice of solvent can greatly influence the type, isomerism and yield of the compounds obtained. Among the possible types of organic reactions, nucleophilic substitution is very common and useful [21]. These reactions are classified as SN1 and SN2 depending on the reaction order of the rate-determining step, unimolecular and bimolecular, respectively. The mechanism for SN1 is associated with the formation of a carbocation as the rate-determining step. The SN2 mechanism is postulated as bimolecular where the transition state is electrically neutral. Therefore, the solvent effect observed for a substitution reaction can help to elucidate their mechanism [22].Figure 2 displays simplified schematics of transition states for SN1 and SN2 which permits a qualitative prediction of rate constants from the relative values of 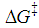 for polar and non-polar solvents:
for polar and non-polar solvents: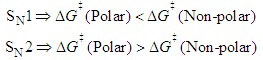 | (4) |
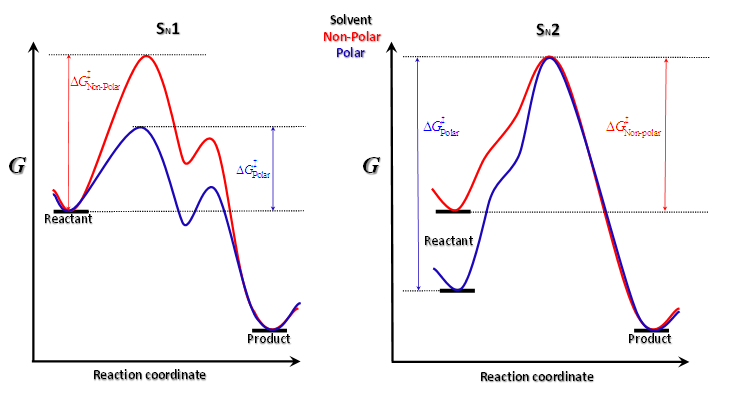 | Figure 2. Simplified schematic plots for the influence of solvent polarity in SN1 and SN2 substitution reaction mechanisms |
2. Objectives
- In this paper, we describe part of a final year undergraduate project, based on the solvatochromic effect performed in the following areas of Physical Chemistry: Characterization by means of solvatochromic probes in different single solvents. Estimation of characteristic parameters that define the Kamlet-Taft and Dimroth–Reichardt solvent scales. Check the validity of these scales in the frame of Linear Free Energy Relationship by using literature data, consisting of pKa and reaction rate constants.The proposed strategy belongs to a wider, more comprehensive, solvatochromic project which was conducted from three different chemical perspectives, viz. Analytical, Physical and Organic Chemistry. The integral project was completed by some final year graduating students that worked on the subject of solvatochromism from these different points of view for a laboratory work of 180 hours [23].
3. Experimental
- UV-Vis spectra were recorded from 350nm to 900 nm for the ET30 probe and from 250nm to 500nm for the other probes, by using a Shimadzu UVmini-1240 Spectrophotometer at 1nm stepwise. A quartz cuvette with a path length 10 mm, and chamber volume 400 μL were employed.a) Solvents:From Panreac [24]: Dimethyl sulfoxide (DMSO) CAS # 67-68-5, N,N-Dimethylformamide (DMF) CAS # 68-12-2 and Tert-butyl alcohol (tBuOH) CAS # 76-65-0 and Methanol (MeOH) CAS # 67-56-1, for analysis; Acetonitrile (CAN) CAS # 75-05-8, Tetrahydrofuran (THF) CAS # 109-99-9 and 2-Propanol (2PrOH) CAS # 67-63-0 for synthesis. From Merck [25]: Acetone (AC), CAS # 67-64-1.b) Solvatochromic probes:From Sigma-Aldrich [26] Reichardt’s dye (ET30), CAS # 10081-39-7, 4-Nitroanisol (4NA) CAS # 100-17-4 and 4-Nitrophenol (4NP) CAS # 100-02-7.c) Molecular sieves:From Sigma-Aldrich, Type 3Å, CAS # 308080-99-1 and Type 4Å, CAS # 70955-01-0.As desiccant, Sodium sulfate anhydrous, Na2SO4, CAS # 7757-82-6 from Merck for analysis, was used.The solvents were desiccated by passing them through a 20x1 cm Na2SO4 column. Then, solvents were stored in amber glass hermetically sealed bottles containing activated molecular sieves type 4Å for DMSO and THF and type 3Å for all other solvents.10mL 1mM ET30 probe in THF was prepared as stock solution. Each ET30 solvent working solutions were prepared adding 20µL ET30 THF stock solution to a 1mL volumetric flask. A sand bath at 40ªC was used to evaporate the THF, and subsequently the solution was made up with the correspondent solvent. In some cases, further dilution would be necessary to maintain the maximum values of absorbance below unity.2mM of 4NA and 4NP (5mL) stock solutions were prepared in each solvent. The working solutions were prepared from dilution to 0.1mM to keep them within the required absorbance range.
4. Results and Discussion
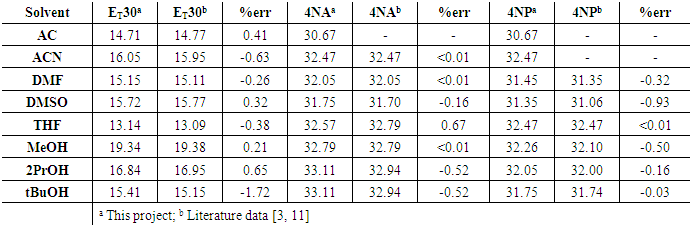
- The Dimroth-Reichardt ET30 polarity scale is based on the λmax value of the lower energy band in the UV-Vis spectrum of Reichardt’s dye in the solvent under study. Figure 3 shows its zwitterion molecular structure of this pyridinium N-phenolate betaine dye.The ET30 energy gap between HOMO-LUMO falls within the visible/near-infrared spectral range, due the high benzene conjugation. Moreover, the large distance between the two charged heteroatoms (see Figure 3) produces a very large molecular dipole moment, in excess of 10 Debye. This high value ensures strong dipolar interaction with other molecules.
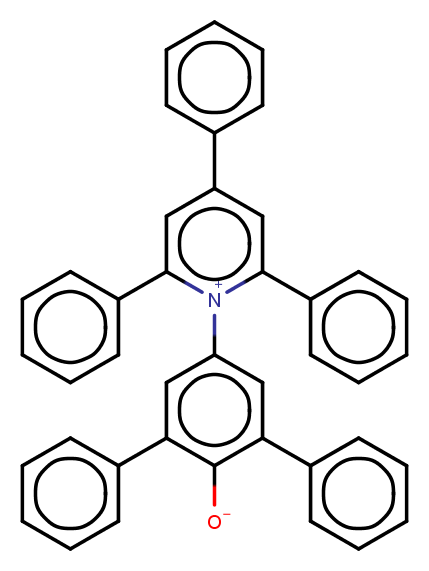 | Figure 3. 2D projection of the zwitterion Betaine ET30 molecular structure |
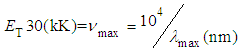 | (5) |
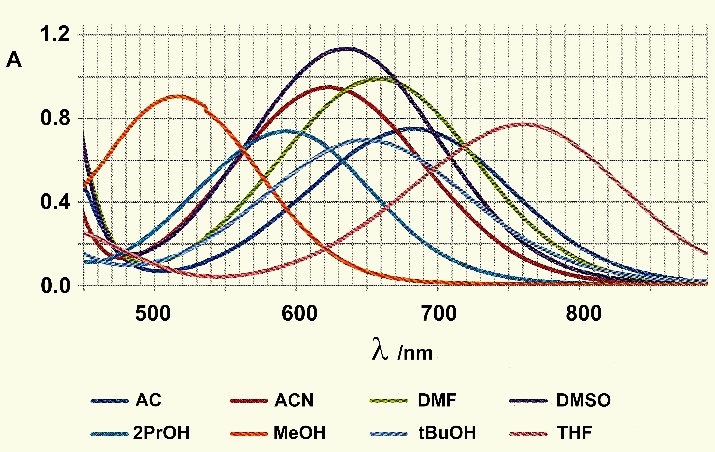 | Figure 4. ET30 visible spectra in the different solvent essays |
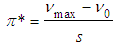 | (6) |
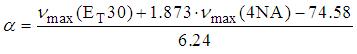 | (7) |
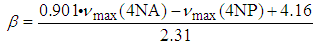 | (8) |
|
|
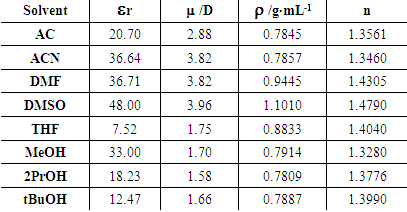
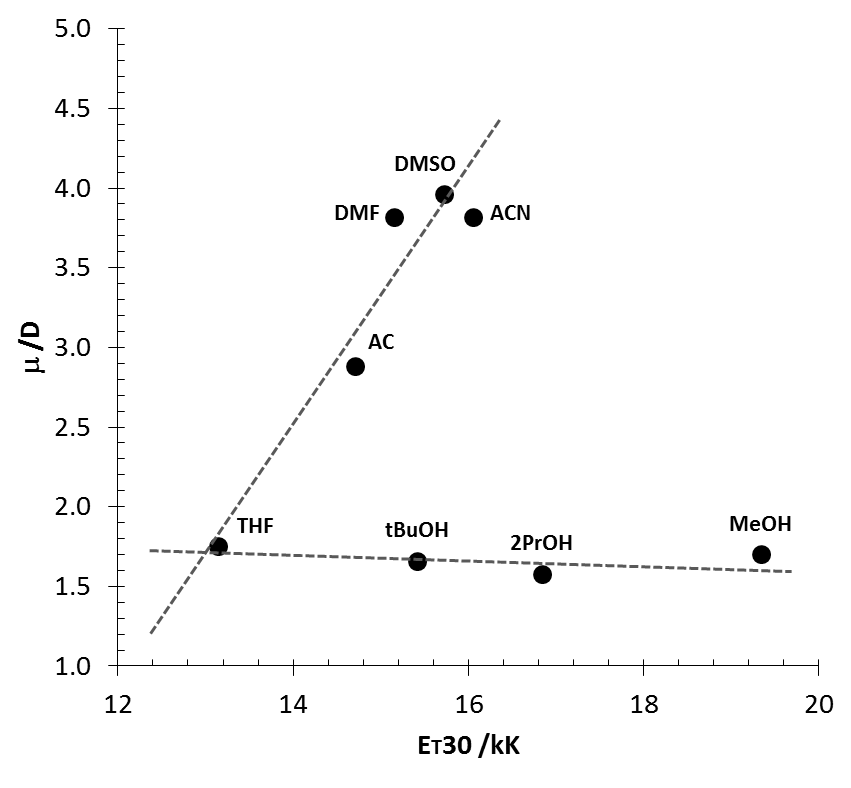 | Figure 5. Plots of solvent dipole moment vs. ET30 polarity scale |
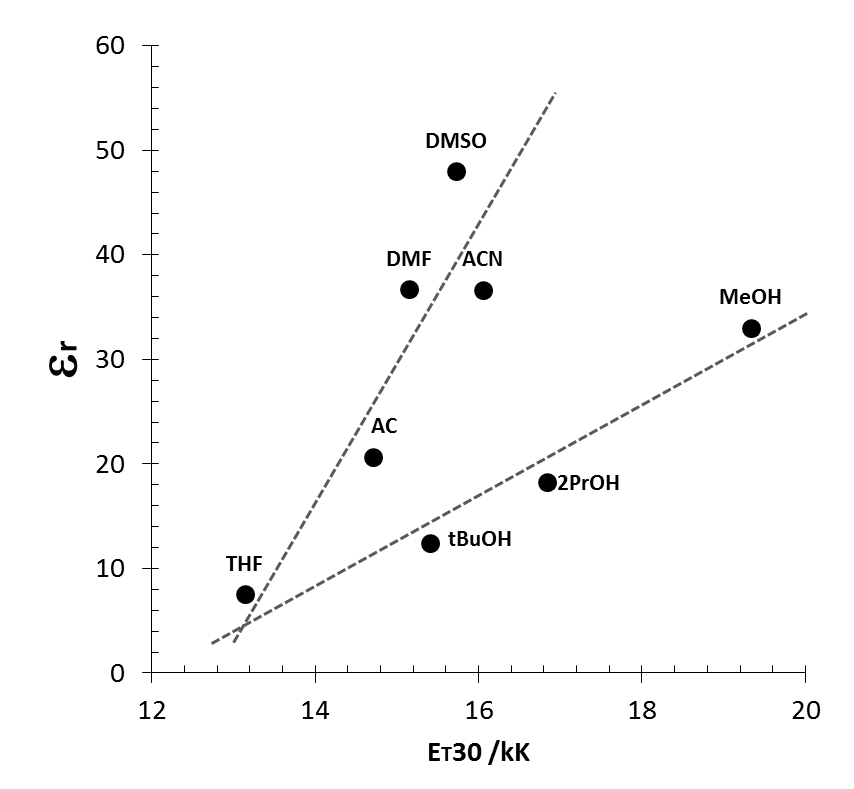 | Figure 6. Plots of solvent relative dielectric permittivity vs. ET30 polarity scale |
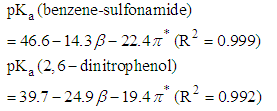 | (9) |
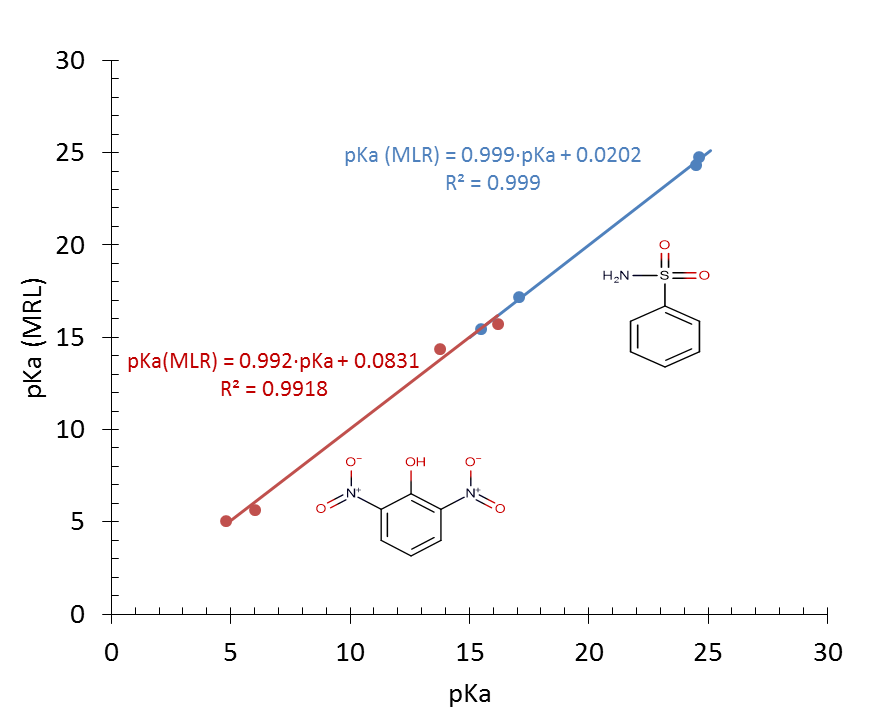
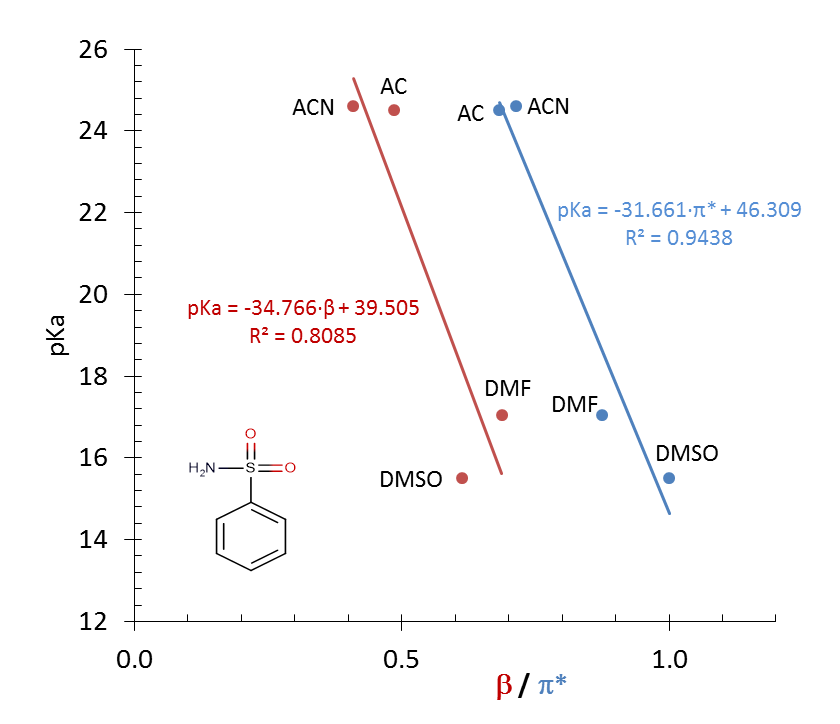 | Figure 7. Linear correlation between benzene-sulfonamide pKa and solvatochromic parameters β (red plot) and π* (blue plot) for the four aprotic solvents |
|
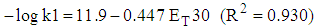 | (10) |
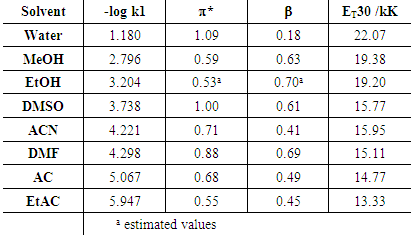
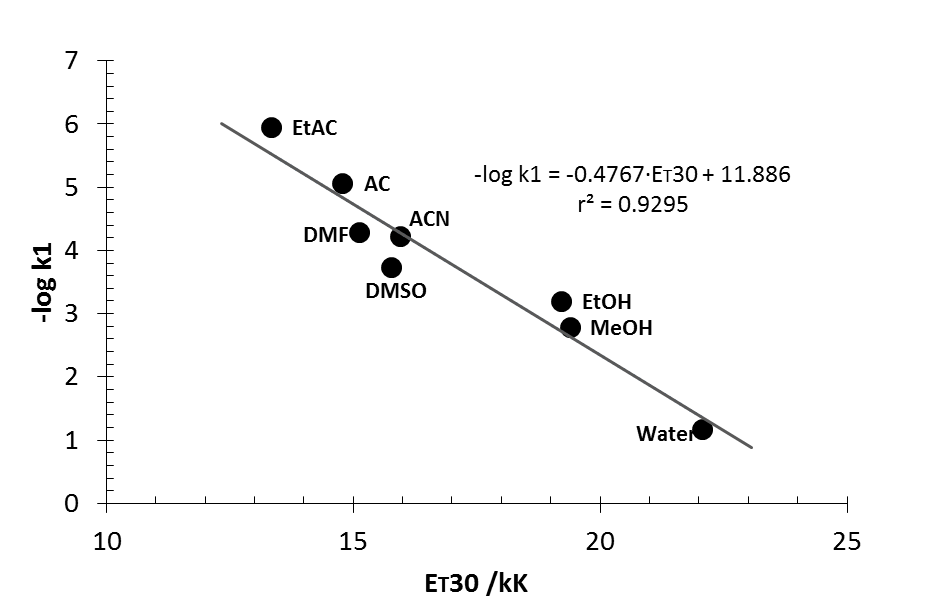 | Figure 9. Plot of relative rates of solvolysis of p-methoxyneophyl p-toluenesulfonate vs. ET30 parameter for different solvents, taken from table XI in ref [30] |
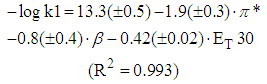 | (11) |
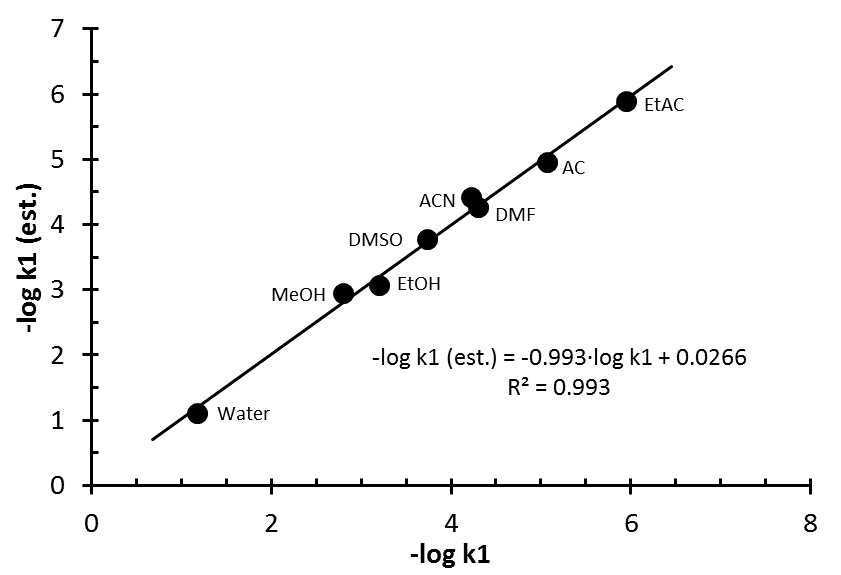 | Figure 10. Linear relationship between solvolysis rate constants estimated and those taken from literature [22] |
5. Conclusions
- The proposed comprehensive “Solvatochromic Project” equips students with a wide-angle view of solvent polarity from different chemical perspectives. Students in the Physical Chemistry section learned about the different hypotheses for solvation and how the use of solvatochromic dyes assists in developing different solvent scales. Within the framework of LFER, solvent effects were analyzed by using literature data of pKa and rate constants of organic substitution reactions, obtaining agreement with hypotheses proposed in the literature. Last but not least, students learned how to recycle and recover the solvents and chemicals they used.
ACKNOWLEDGEMENTS
- The authors are very grateful to Prof. W.H. Mulder for his helpful comments and suggestions. We also thank the following students of Chemistry: R. Cabezas Rodríguez, A. Martínez Ceballos and M.V. Sánchez Micó for their enthusiasm and dedication in developing part of the “Solvatochromic Project”.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML