-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
p-ISSN: 2331-7450 e-ISSN: 2331-7469
2016; 4(2): 38-44
doi:10.5923/j.jlce.20160402.03

Follow in Rudolf Diesel Footsteps: Introducing High School Students to Microwave-assisted Preparation of Biodiesel from Peanut Oil
Luzia P. Novaki1, Ana M. Chinelatto1, Maria J. L. d’Almeida e Silva2, Paula P. Brotero3, Omar A. El Seoud1
1Institute of Chemistry, the University of São Paulo, Brazil
2Lectus Education System, Alameda dos Aicás, São Paulo, Brazil
3São Paulo Waldorf-Micael High School, São Paulo, Brazil
Correspondence to: Omar A. El Seoud, Institute of Chemistry, the University of São Paulo, Brazil.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The term biodiesel, BD, denotes the fuel composed of mixture of fatty acid monoesters (usually methyl or ethyl) obtained by transesterification of oils and fats.We developed a three-stage project for twelfth grade high school students on microwave (MW)-assisted preparation of BD and its analysis. We used this project to introduce new concepts (catalysis; quantitative analysis) and techniques (use of MW in chemistry; product separation from mixtures), and promote contact of high school students with the university. The first stage, carried out in the school, involved literature survey on BD, and laboratory purification of BD samples that we prepared at the Institute of Chemistry of the University of São Paulo, Chem-USP. In the second-stage, the students visited Chem-USP, where we demonstrated MW-assisted transesterification of peanut oil, PNO. Then they participated in the following experimental activities: (i) analysis of PNO-based BD by gas chromatography; (ii) determination of the refractive indices and densities of the reactants (ethanol and PNO) and products (glycerol and BD) of the transesterification reaction; (iii) investigating the dependence of MW-heating of ethanol-isooctane mixtures on their ethanol content. The third stage was carried out at the school where the students presented/discussed their results, and evaluated the project. The experimental part of the project is simple, safe, low-cost, generates little waste, and can be carried out in a typical high school laboratory.
Keywords: Biodiesel preparation, Peanut oil, Microwave-assisted reactions, Gas chromatographic analysis, Active learning, Constructivist teaching
Cite this paper: Luzia P. Novaki, Ana M. Chinelatto, Maria J. L. d’Almeida e Silva, Paula P. Brotero, Omar A. El Seoud, Follow in Rudolf Diesel Footsteps: Introducing High School Students to Microwave-assisted Preparation of Biodiesel from Peanut Oil, Journal of Laboratory Chemical Education, Vol. 4 No. 2, 2016, pp. 38-44. doi: 10.5923/j.jlce.20160402.03.
Article Outline
1. Introduction
- Biofuels are produced from organic material, including plant- and animal-based feedstock, often referred to as “biomass”. Here we are interested in the liquid fuels prepared from vegetable oils that are commercially used as blends with petroleum-based fuels. After patenting his internal compression (diesel) engine in 1892, Rudolf Christian Karl Diesel ran his motor on peanut oil (PNO; 1900), and later (1911) wrote ‘‘the diesel engine can be fed with vegetable oils and would help considerably in the development of agriculture of countries which will use it.’’ Although feasible, the use of vegetable oils as diesel fuel is not convenient because of their relatively high viscosity and flash point; the latter makes the ignition relatively difficult. These technical problems were solved by using biodiesel (BD) which is defined as a clean-burning mixture of (usually) methyl or ethyl esters of fatty acids, obtained by acyl-transfer reaction (transesterification; see scheme 1) of natural, renewable feed stock, such as vegetable oils and animal fats. [1, 2]
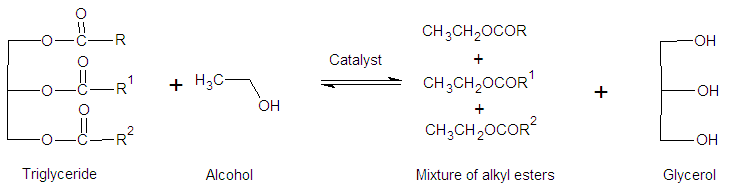 | Scheme 1. General scheme for transesterification of a vegetable oil (triglyceride) with an alcohol (usually methanol or ethanol) in the presence of acid or base catalyst |
2. Experimental Part
2.1. Solvents, Reagents, and Peanut Oil
- All chemicals were purchased from Merck and were purified, where appropriate, as given elsewhere [13]. We purchased PNO (Esperança, 900 mL bottle) from a local supermarket.
2.2. Equipment
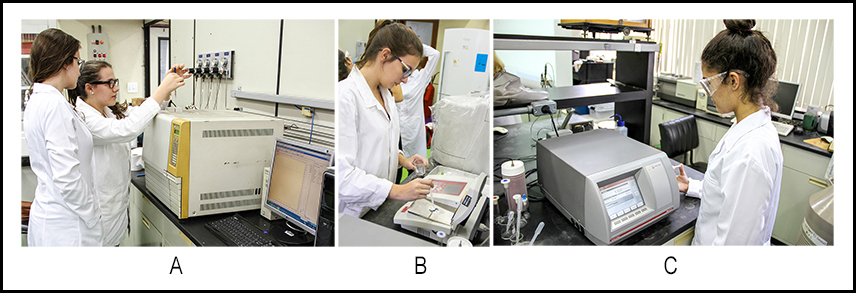
- The students used the following equipment at Chem-USP: DMA 4500M digital, resonating density meter (Anton-Paar; d ± 0.00001 g. cm-3), equipped with a closed reservoir for collecting the spent sample after measurement; J357 digital refractometer, operating at 488 nm (Rudolph Research; n ± 0.00001). Both equipment are provided with Peltier thermoelectric heat pumps for temperature control, within 0.01°C. Gas chromatographic analysis was carried out with Shimadzu GC-17A equipment. The analysis conditions were: Supelcowax-10 capillary column, OD = 0.25mm, stationary phase layer thickness = 0.25μm, length = 30 meters; FID detector; helium gas (mobile phase); sample split ratio 100:1; starting column temperature, T = 200°C for 2 minutes, followed by T increase at 10°C/minute for 4 minutes, and then isothermal heating at 240°C. The temperature of ethanol-isooctane mixtures before and after WM-irradiation was measured with the thermocouple sensor of EA-15 digital thermometer (Extech Instruments; Figure 1A). The thermometer temperature sensor was placed in a short NMR tube (OD = 5mm) containing silicon oil for thermal conductance.
2.3. Synthesis of Ethyl Carboxylate References for Gas Chromatographic Analysis
- Ethyl undecanoate and ethyl tridecanoate were prepared by reaction of the appropriate acid (0.1 mol) with anhydrous ethanol (50 mL), in the presence of concentrated sulfuric acid catalyst (1.5 ml), under reflux for 6 h. Excess ethanol was removed under reduced pressure; the resulting liquid was washed with cold, saturated NaCl solution until the aqueous phase was neutral, and then dried with granular anhydrous MgSO4. The esters were purified by fractional distillation under reduced pressure. Yield 70 and 75%, respectively; ethyl undecanoate 105°C/4 mmHg; ethyl tridecanoate 121-123 C/2.2 mmHg [14]. Their purity was found to be > 99,9% as shown by gas chromatographic analysis, vide infra.
2.4. Microwave-assisted Preparation of Biodiesel from Peanut Oil
- A solution of 0.75 wt% sodium ethoxide/ethanol was prepared by dissolving clean sodium metal in the appropriate volume of anhydrous ethanol at 60°C (caution). For the transesterification reaction, we heated a mixture of 20g PNO and 50 mL of 0.75 wt% sodium ethoxide/ethanol in 150 mL reactor flask, equipped with magnetic stirring bar, and inserted in the cavity of Discover MW-oven (CEM). The mixture was subjected to 100W MW-irradiation power for 15 minutes, see part (A) of Figure 1. We removed excess ethanol under reduced pressure; the product separated into two-phases; we sent these mixtures to the schools for purification of the produced BD. The students purified the latter by separating the phases (lower glycerol, upper BD); washing the upper one with cold, saturated NaCl solution until the aqueous phase was neutral, followed by drying the BD over granular anhydrous MgSO4, and filtration. Yields 65-75% of pale yellow oil, see Figure 2.
 | Figure 2. From left, peanuts; peanut oil and peanut-based diesel oil |
2.5. Gas Chromatographic Analysis of Peanut Oil-based Biodiesel
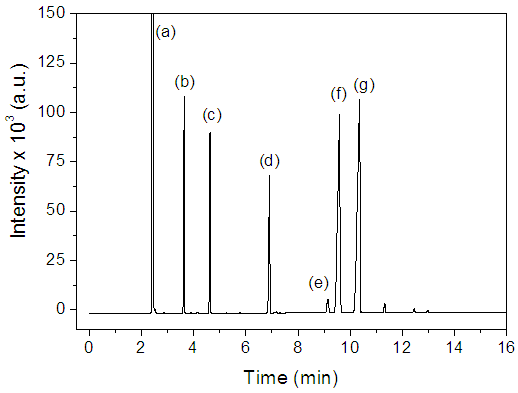
- We prepared a mixture of 150 µL BD, 20 µL of ethyl undecanoate, 20 µL of ethyl tridecanoate (these two esters serve as internal references) and completed the volume to 2 mL with dry ethanol. The students analyzed this mixture by injecting 1 µL sample of the alcoholic solution into Shimadzu GC-17A equipment under the conditions listed in item 2.2; the total experiment time was ca. 20 minutes; part (A) of Figure 3.
2.6. Measuring the Refractive Index and Density of Reactants and Products of the Transesterification Reaction
- The students measured the densities and the refractive indices of the reactants (PNO and ethanol) and products (glycerol and biodiesel) using the equipment mentioned in 2.2; parts B and C of Figure 3, respectively. All measurements were at 25°C.
2.7. Effect of Ethanol Volume Percentage, %VEthanol, on the Efficiency of Microwave Heating of Ethanol-isooctane Mixtures
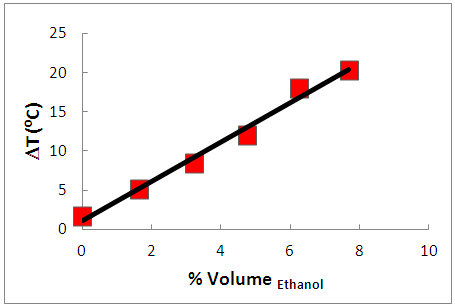
- The students added 60 mL isooctane to each of two flasks A and B; placed both in the water bath for T equilibration at 25 ± 0.2°C (caution: use lead donut flask stabilizers), and measured the temperature of the liquid before MW-irradiation. They placed one flask in the cavity of the above-mentioned MW-oven, and heated the isooctane for one minute with 150 W irradiation power. They removed the flask from the MW-oven, quickly measured the liquid temperature, and put it back in the water bath for T equilibration. They repeated the same procedure after adding ethanol to isooctane, as follows: Flask (A) 1mL, then 2mL, then 2mL ethanol (cumulative added volumes of ethanol = 1mL, 3mL, and 5mL alcohol, respectively); flask (B) 2mL, then 2mL, then 2mL ethanol (cumulative added volumes of ethanol = 2 ml, 4 mL, and 6 mL alcohol, respectively). After each MW-irradiation, the students removed the flask from the MW-equipment, quickly measured and recorded the liquid temperatures in a table that showed the dependence of ΔT (= Final liquid temperature - its temperature before MW irradiation) versus %VEthanol in the binary solvent mixture. Assuming ideal mixing, the final binary mixture volume = VIsooctane + VEthanol.
3. Results and Discussion
3.1. Choice of the Vegetable Oil
- We were motivated to use PNO because of the above-mentioned historic note on its use by Rudolf Diesel in his revolutionary engine. Although the domestic use of PNO was largely surpassed by soybean and sunflower oils, it is still employed in cooking and salad dressing. As edible oil, PNO is healthy because its fatty acid composition is similar to other extensively used oils, including rapeseed, soybean, and sunflower [15-18].
3.2. First Stage: Literature Survey on BD and Its Purification in the Laboratory
- As an assignment, the teacher asked the students to survey the literature on the definition and production of BD. Concomitantly, the students went to the laboratory where they separated BD from the transesterification reaction mixtures that we sent, as given in 2.4 of Experimental.
3.3. Second Stage: Experimental Work at Chem-USP
- Three student groups visited our laboratory at Chem-USP. In each visit, we showed the students short videos about: MW-heating [19]; the steps of the transesterification reaction of PNO (video prepared by us); principles of GC analysis (power point presentation based on reference [20]); this activity took 30 minutes.The students were then divided into three groups; they “rotated” between the following three experiments: (i) determination of the composition of the BD that they purified using GC (part (A) of Figure 3; GC results shown in Figure 4); (ii) determination of the refractive indices and densities at 25°C of ethanol, PNO, purified BD, and glycerol (parts (B) and (C) of Figure 3, respectively); (iii) measurement of the dependence of MW-heating of ethanol-isooctane mixtures on VEthanol (part (B) of Figure 1); these activities took three hours. In the last half an hour, we joined the groups, summarized the activities done, and plotted the graph of ΔT versus %VEthanol, Figure 5.
3.4. Third Stage: Discussion of the Results and Evaluation of the Project
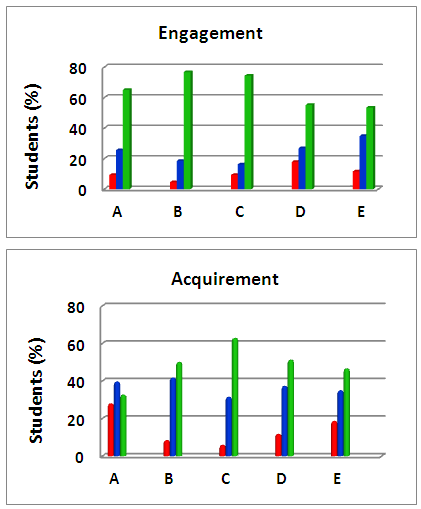
- In the third stage, the students presented and discussed their results in class, and evaluated the project. The discussion of CG results included the concept of using internal references for qualitative analysis; fatty ester composition of BD (hence fatty acid composition of PNO), and a comparison between the compositions of PNO and soybean oil [22]. Integration of the peaks of Figure 4 gave the following composition for PNO (w/w): 12.9% ethyl hexadecanoate; 2.3% ethyl octadecanoate; 39.0% ethyl 9Z-octadecenoate (ethyl oleate); and 45.8% ethyl 9Z, 12Z-octadecadienoate (ethyl linoleate). This composition is not far from that reported elsewhere for PNO [23]; the composition of a Brazilian soybean oil sample is (w/w): 11.8% (ethyl hexadecanoate); 3.2% (ethyl octadecanoate); 22.3% (ethyl oleate), and 53.5% (ethyl linoleate) [22].The students observed the differences between density (D, g/cm3) and refractive index (RI) of reactants and products of the transesterification reaction. The results of one group were: PNO (D = 0.9147; RI = 1.47769); ethanol (D = 0.8008; RI = 1.36636); biodiesel (D = 0.8897; RI = 1.46154) and glycerol (D = 1.2560; RI = 1.46819); similar data were recorded by the other groups. Based on these results, the students suggested the use of RI as a simple technique to follow the progress of the transesterification reaction. Their teachers explained, however, that the difference of RI between reactants and products is a required, but may not be sufficient condition for using this technique directly. The reason is the presence of glycerol and, particularly, excess ethanol. Thus this technique was successfully employed to follow transesterification after phase separation by centrifugation and ethanol removal [24], or from calibration curves between RI and ester content, using authentic fatty acid esters [25]. The mechanism of MW-heating was briefly discussed [26]. The ethanol molecule aligns its dipole with the electromagnetic field, generated by the oven´s magnetron (the vacuum tube that generates the MW frequencies). As the applied field oscillates (at 2.45 GHz) the ethanol dipoles attempts to realign themselves with the alternating electric field. During this process, energy is lost in the form of heat through molecular friction and dielectric loss. The ability of a substance to convert microwave energy into heat at a given frequency and T is determined by the so-called loss tangent (tan δ) whose values are 0.941 for ethanol (efficient MW-absorption/heating) and 0.02 for n-hexane, a typical aliphatic hydrocarbon (negligible MW-absorption/heating). This large difference in (tan δ) between ethanol and isooctane explains the negligible heating of isooctane and the linear increase in ΔT as a function of increasing % VEthanol in the binary mixture. After this discussion, a project evaluation sheet was handed out. Figure 6 summarizes the students´ answers regarding their participation in the different stages of the project.
4. Conclusions
- The aim of the present project goes beyond performing a new experiment. We introduced new concepts and techniques, and linked theory to experiment. We promoted the contact of high school students with the university by carrying out the project in consecutive stages that involved both institutions. The students purified BD in their schools and then used the results of CG analysis to determine the fatty acid composition of BD, hence of PNO. We recommend this project because of the socio-economic relevance of the subject and the new concepts and techniques learned. Additionally, the project is safe, low-cost, and can be carried out in any reasonably equipped high school laboratory. Regarding safety, handling sodium metal is not required, as the transesterification experiment can be done with aqueous NaOH solution as a catalyst instead of sodium ethoxide [4]. Additionally, convection heating can be employed in transesterification if a scientific MW-oven (not household one) is not available in the school [27]. Although the use of digital densimeter and refractive index equipment is convenient and expedient, the same results can be obtained by using volumetric glassware, analytical balance, and Abbe refractometer, respectively. There are many videos on GC, e.g., [28]. The BD composition can be analyzed in an outside laboratory if a GC equipment is not available in the school. Most importantly, the project serves to introduce new concepts (MW interaction with matter; quantitative analysis) and techniques (MW-assisted synthesis; GC analysis) and linked the students to an everyday situation, the use of BD as a renewable fuel.
ACKNOWLEDGEMENTS
- We are grateful to the students who participated in this project, to Gabriel B. Polho and Pedro L. Furlam (School of Medicine-USP) for their help during the São Paulo “School of Chemistry”. O. A. El Seoud thanks FAPESP (grant 2014/22136-4) and CNPq (grant 307022/2014-5) for financial support and for research productivity fellowship to O. A. El Seoud, respectively.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML