-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
2015; 3(4): 76-80
doi:10.5923/j.jlce.20150304.03

Synthesis of Sulfanilamide Derivatives and Their Effects on E. coli and B. subtilis: A Collaborative Experiment between Undergraduate Organic and Microbiology Laboratories
Corey Stilts, Christine Bezotte, Sue Fontaine
Division of Mathematic and Natural Sciences, Elmira College, Elmira, USA
Correspondence to: Corey Stilts, Division of Mathematic and Natural Sciences, Elmira College, Elmira, USA.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In this experiment, organic chemistry students synthesized derivatives of their own design of the antibiotic sulfanilamide. The student designs varied from direct modification of sulfanilamide to variations in the preparation of sulfanilamide. After optimizing their synthesis, the organic groups shared their products with the microbiology lab students. The microbiology students used the Kirby-Bauer antibiotic testing method. The students analyzed the derivatives designed by the organic students versus the control antibiotic sulfanilamide. The students used impregnated filter disks on the bacteria Escherichia coli and Bacillus subtilis to measure the zone of inhibition after a 24-hour period. The project ended with a joint poster session between the organic and microbiology labs. Students discussed their results and compared them to the control antibiotic sulfanilamide. The experimental results for the student designed derivatives gave zones of inhibition that were comparable to that of sulfanilamide.
Keywords: Organic Chemistry, Collaborative Learning, Cooperative Learning,Inquiry-based, Microbiology, Laboratory Instruction, Multidisciplinary, Antibiotics, Organic Synthesis, Laboratory Experiment
Cite this paper: Corey Stilts, Christine Bezotte, Sue Fontaine, Synthesis of Sulfanilamide Derivatives and Their Effects on E. coli and B. subtilis: A Collaborative Experiment between Undergraduate Organic and Microbiology Laboratories, Journal of Laboratory Chemical Education, Vol. 3 No. 4, 2015, pp. 76-80. doi: 10.5923/j.jlce.20150304.03.
Article Outline
1. Introduction
- Understanding and developing connections across the scientific disciplines is a necessary part of any undergraduate science major. In a Cell Biology Education article, Joan Steitz comments on a text by the National Academies’ Committee on Undergraduate Education to Prepare Research Scientists for the 21st Century [1], [2] She explains the fourth recommendation by the committee as, “Laboratory courses should be as interdisciplinary as possible, since laboratory experiments confront students with real-world observations that do not separate well into conventional disciplines.” This lab describes a collaborative project between organic chemistry II lab students and students in our microbiology lab. A previous collaborative lab involved the synthesis of auxin derivatives and how they affect the growth of Ceratopteris richardii [3]. This interdisciplinary collaboration focuses on the synthesis of sulfanilamide derivatives and the determination of their antibiotic effects. The synthesis portion of the experiment was carried out by sophomore level organic chemistry II lab students. The antibiotic properties of the derivatives were determined by our junior level microbiology lab. They examined the effects of the derivatives on the bacteria Escherichia coli and Bacillus subtilis using the Kirby-Bauer method [4]. At the end of the semester, students discussed their results and presented their research in a joint poster session.Team teaching has been a component of the chemistry and biology courses for a number of years. Within both disciplines we have found the collaborative experiments to be the most rewarding for the students and the instructors. The organic students enjoyed having ownership of their own projects as well as having the opportunity to see the effectiveness of their derivatives as antibiotics. The microbiology students enjoyed analyzing the derivatives by their fellow students and seeing the effects small changes to the structure of a drug can make. We also have had students who were taking both courses at the same time and were able to participate in the entire experiment and evaluate their own synthesized derivatives.
2. Materials and Methods
2.1. Experimental Overview
2.1.1. Organic Experimental Setup
- The synthetic part of the experiment was designed for a second semester organic laboratory and the students in this lab are traditionally sophomores. Our lab size is eighteen students and the students were divided into six groups of three. They were given the first six weeks of the semester to design and synthesize a derivative of sulfanilamide. Examples of two successfully synthesized derivatives 4-A-35DCBS and 4-AB-1-SC and the control (sulfanilamide) are given in Figure 1. In the first week, we gave the groups some background information on sulfa drugs and how they affect bacterial growth. We also gave them a procedure for the synthesis of sulfanilamide [5] and other resources to help them with their syntheses [6, 7]. The two most common approaches by the students were modification of the sulfanilamide synthetic scheme or a modification of sulfanilamide itself.
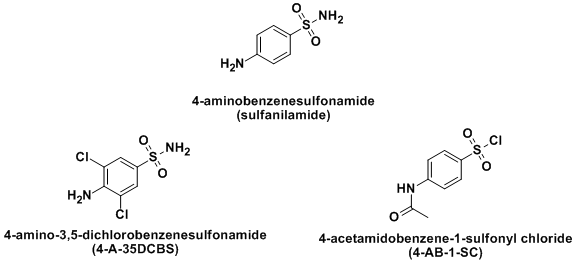 | Figure 1. Structure of sulfanilamide and examples of student derivatives (4-A-35DCBS and 4-AB-1-SC) |
2.1.2. Microbiology Experimental Setup
- Our microbiology lab used the derivatives from the organic lab to evaluate their effectiveness along with commercial grade sulfanilamide on the bacteria E. coli (Gram -) and B. subtilis (Gram +) using the Kirby-Bauer Method. The derivatives were added to sterile filter disks in three different volumes (5,10, and 50 μL) and placed on a freshly inoculated lawn of each bacterium. The bacteria were allowed to incubate for 48 hours and then the zone of inhibition was measured and recorded. The students used data from three trials and used it to create an average zone of inhibition for each derivative and sulfanilamide. This portion of the experiment was much shorter than the organic portion. It can be accomplished in one lab period with some follow-up time required for discussion and measurements. In class, microbiology students were studying the characteristics, effects and properties of antibiotics. For the lab, all students were provided with further background information on sulfa drugs and their mechanism of action as well as the Kirby-Bauer procedure [8]. At the end of the semester student groups presented their experiments in group poster sessions. The poster sessions were scheduled at a time during the week that all students involved in the project could attend.
2.1.3. Altering Experimental Setup for Larger Classes
- The size of the organic lab section and the microbiology lab sections each have about 15-20 students. Since the synthetic part of the experiment is open-ended (students are given basic instructions and requirements), this lab may have to be modified to facilitate larger groups and reduce the number of lab periods. Each group of three worked on their own individual project. The budget for this project was no more than the budget for the course when we had weekly syntheses. The cost for the entire 6 week course for 20 students was ~$600 or about $5 per student per week. Even though the students had individual projects and each group needed their own set of reagents, there were many reagents that overlapped all groups. Also, because this was a six-week project, we did not have to purchase the normal reagents that would be required for those weeks if we were using the traditional weekly organic lab curriculum.The experiment was developed using a class of 28 organic students that were divided into lab sections and one section of 17 microbiology students. In order to accommodate larger section sizes and/or a larger number of sections, it may be necessary to alter the openness of the organic chemistry portion of the lab. The microbiology lab is a standard Kirby-Bauer lab and is completed in one-week and would not have to be altered for a larger group. Some possible alterations to the organic chemistry portion of the lab could include: 1) Create pre-planned syntheses for all students 2) Make it so that each section of lab was responsible for the synthesis of one derivative 3) Increase the number of weeks in the lab
2.2. Synthesis of the Sulfanilamide Derivatives in the Organic Chemistry II Lab
- The experimental portion took place in the first six weeks of the organic II chemistry lab. During the first two weeks the students were given an overview of sulfa drugs and their actions. During this time, they also developed their synthesis and collected all reagents. This time frame also allowed the instructor’s time to order any reagents required for the projects. The students were given the basic requirements of their synthesis. The three basic requirements of the derivatives were:1) The derivative must be based on sulfanilamide2) The compounds must be soluble in dimethyl sulfoxide or water3) All procedures must be safe and approved by the instructorThey also had to provide enough of their final product so that it could be used by the microbiology students. The goal for each group was to make 0.5 grams of their derivative. The students were also given the responsibility of finding an appropriate synthesis and determining what reagents they might need. Most of the students found the first two weeks the most difficult as they were used to having a procedure already prepared for them and all the reagents prepared for their use. This planning phase provided students with opportunity for library research of previous work and methods to scale up a reaction. Students then developed their own synthetic schemes based on modifications of previous syntheses. Most of these opportunities are not provided in a traditional “cookbook” style approach to learning organic chemistry. All students were required to provide a MSDS sheet for all reagents they were using as well as any safety precautions that were specific to their procedure. All reagents used in the experiments were purchased through the Aldrich Chemical Company.All of the students were provided with a general synthesis for sulfanilamide. The general synthesis can be seen in Figure 2. Many of the students chose this synthesis as their starting point in the creation of their own synthetic scheme. They tried the substitution method in which they would change a reagent in the synthetic scheme to change the structure of the final product. Since we had sulfanilamide as a reagent in our chemical stockroom, the groups that chose not to alter the sulfanilamide synthetic pathway chose to start with sulfanilamide and modify it directly.
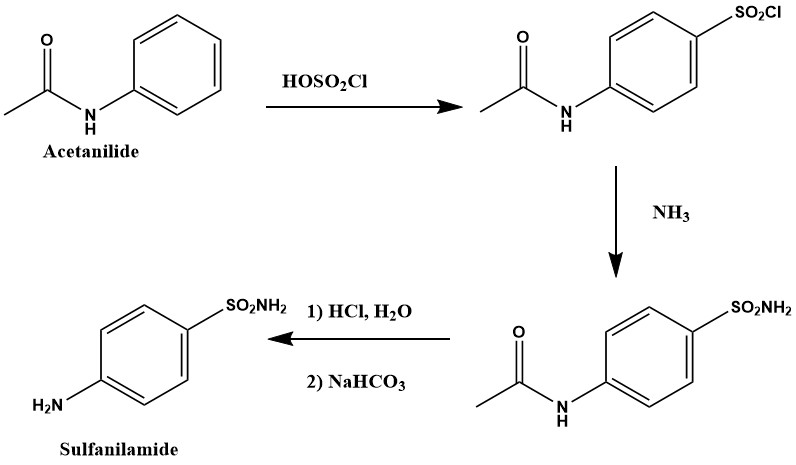 | Figure 2. Synthesis of sulfanilamide |
2.3. Analysis of the Sulfanilamide Derivatives in the Microbiology Lab
- We used the Kirby Bauer disk diffusion method to analyze the effectiveness of the derivatives. All bacteria (E. coli and B. subtilis) were first grown in Tryptic Soy Broth (TSB) overnight at 37oC for use in lab the next day. The students were given 9 agar plates that had already been prepared and sterile filter disks (1cm diameter). Using sterile swabs the students made three lawns each of E. coli and B. subtilis. The students were provided with the sulafanilamide derivatives and sulfanilamide (control) dissolved in dimethyl sulfoxide (DMSO) with a concentration of 1g/mL. The antibiotics were absorbed into the sterile disks, by volume. Each plate had three filters disks placed on them. The disks were spaced evenly apart. Antibiotic disks were distributed onto the fresh inoculated lawns so that each plate with its three evenly spaced filter disks received a dose of 5,10 and 50μL of antibiotic (See Table 1). The antibiotics were administered via micropipette to the center of each disk. The plates were labeled and stored in the lab and incubated at 37OC for two days. After the incubation period, the students recorded the zones of inhibition for each filter disk and recorded the distances. A sample of the recording chart is available in the supplemental information. All supplies for the microbiology lab were purchased through the Ward’s science.
3. Results and Discussion
3.1. Synthesis of the Sulfanilamide Derivatives
- As in most organic chemistry labs we have had mixed results with the syntheses. The students generally chose one of two pathways for their synthesis. They chose to modify the synthetic pathway of sulfanilamide or they started with sulfanilamide and modify its structure. We provided the students with a variety of synthetic texts and lab manuals. The students had synthetic yields that varied from 20% to 85%. Our lab group class was composed of 5 groups of three. Of those 5 groups, only two groups produced compounds that were used in the studies (4-A-35DCBS and 4-AB-1-SC). In all cases the students were able to research, design and troubleshoot their own synthetic schemes. However, some groups were either unable to isolate enough product or the products were not sufficiently soluble for the microbiology study. For those students who were having trouble, we allowed them to synthesize the control sulfanilamide according to the procedure in the laboratory textbook [5].An example synthesis, the synthesis for 4-A-35DCBS can be seen in figure 3 [6].
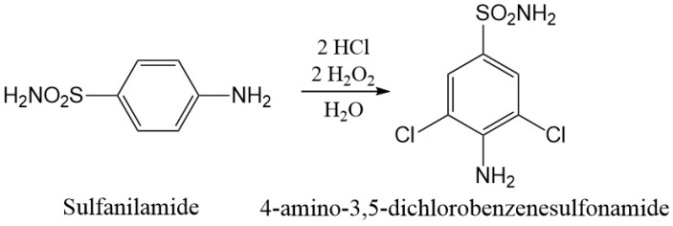 | Figure 3. Synthesis of the derivative 4a-35-DCBS |
3.2. Analysis of the Derivatives by the Kirby-Bauer Method
- After 48 hours, the students recorded the zone of inhibition created by each filter disc by using a ruler to measure the distance from the filter disc to the bacterial colonies. The distances were measured in mm. A sample Kirby-Bauer plate can be seen in figure 4. The students collated their individual results and created the graphs seen in figures 5 and 6. Results for all groups followed the same pattern.
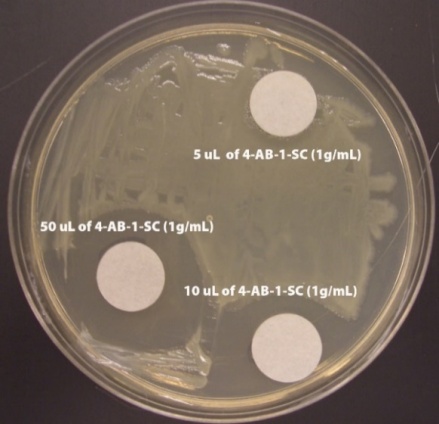 | Figure 4. Example Kirby-Bauer Plate showing zones of inhibition for 4-AB-1-SC |
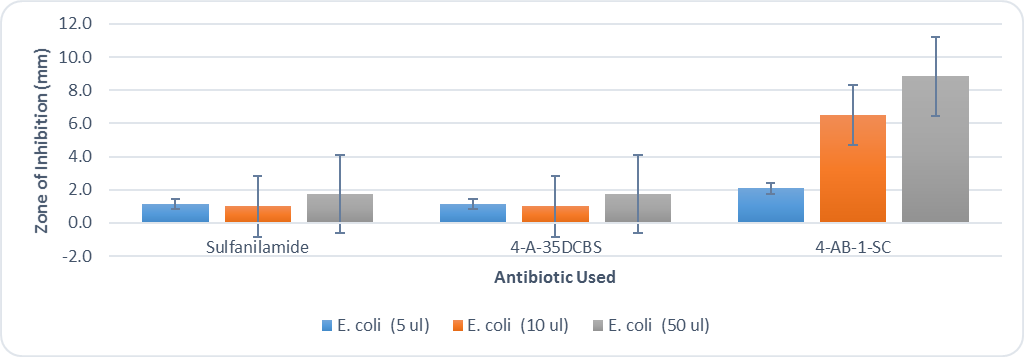 | Figure 5. Kirby Bauer results for E. coli using sulfanilamide and the derivatives 4-A-35DCBS and 4-AB-1-SC |
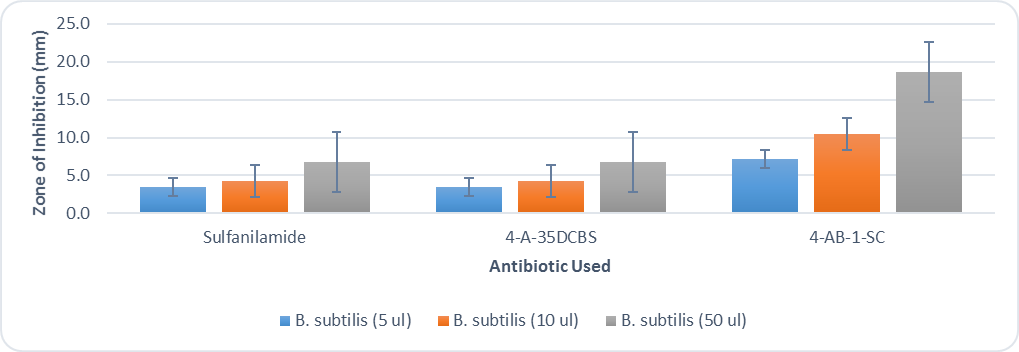 | Figure 6. Kirby Bauer results for B. subtilis using sulfanilamide and the derivatives 4-A-35DCBS and 4-AB-1-SC |
3.3. Poster Session
- At the end of the semester, after both experiments had been completed, the students presented a joint poster session. Each poster included an abstract, introduction, materials, methods, results and discussion. The posters were aligned so that the organic group who synthesized the derivative could stand beside the microbiology group that did the analysis on their derivative. The organic student group presented their poster first followed by the microbiology group. The session was organized like a gallery walk in that the entire class moved from poster to poster to hear the presentations. All the data from the experiments was posted on the Angel (course management system) prior to the poster session. This gave the students the opportunity to see the results from other groups. This allowed them to compare and contrast their results to the other groups and to see any changes in effectiveness and how structure affects activity. A rubric for the poster session is available in the supplementary materials.
4. Hazards
4.1. Organic Chemistry Lab
- Since every lab group had their own specific syntheses, they each met individually with the instructor to discuss the hazards associated with their reactions. All students followed general lab safety procedures (safety glasses, gloves etc.) and were under the supervision of their instructor during the lab. All chemical waste was disposed of in accordance with the college chemical hygiene plan and the MSDS for each individual reagent.
4.2. Microbiology Lab
- The students in the Microbiology lab used aseptic techniques to transfer bacterial colonies and proper chemical safety (safety glasses, gloves etc.) was used in the applications of the antibiotics. After the experiment, all cultures were autoclaved and discarded. All chemical waste was disposed of in accordance with the college chemical hygiene plan and the MSDS for each individual reagent.
Supplemental Materials
- The supplemental materials provide background information on sulfa drugs, classroom handouts, material lists, synthetic procedures and grading rubrics for the poster session.
ACKNOWLEDGEMENTS
- We’d like to thank our CHE2O20 (Organic Chemistry II) and BIO3050 (Microbiology) students for testing this experiment and providing us with valuable feedback.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML