-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
2015; 3(3): 53-58
doi:10.5923/j.jlce.20150303.03
Interdisciplinary Teaching Strategy in the Development of Chemical Courses
Nancy Romero-Ceronio, Carlos E. Lobato-García, Abraham Gómez-Rivera, Ammy J. Gallegos-García, Domingo J. Velázquez-Oropeza
División Académica de Ciencias Básicas, Universidad Juárez Autónoma de Tabasco, Cunduacán, Tabasco, México
Correspondence to: Nancy Romero-Ceronio, División Académica de Ciencias Básicas, Universidad Juárez Autónoma de Tabasco, Cunduacán, Tabasco, México.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Today interdisciplinary teaching and research are already strong and important aspects requested for solving questions. In this context, individual disciplines do not consider all the aspects of a complex problem and interdisciplinary courses and programs may help in facilitating or enhancing the transfer of higher-order cognitive skills (HOCS) such as: critical thinking, problem solving, decision-making and laboratory practice. In this paper, we report a systematic integration of three undergraduate chemistry courses: Chemistry of Natural Products (QPN), Laboratory of Organic Chemistry 2 (LQO2), and Laboratory Analytical Chemistry 1 (LQA1). By choosing a common topic, we prepared a series of activities focused in interdisciplinary interaction and the development of students’ HOCS.
Keywords: Interdisciplinary, Teaching's strategy, Collaborative learning and higher-order cognitive skills (HOCS)
Cite this paper: Nancy Romero-Ceronio, Carlos E. Lobato-García, Abraham Gómez-Rivera, Ammy J. Gallegos-García, Domingo J. Velázquez-Oropeza, Interdisciplinary Teaching Strategy in the Development of Chemical Courses, Journal of Laboratory Chemical Education, Vol. 3 No. 3, 2015, pp. 53-58. doi: 10.5923/j.jlce.20150303.03.
Article Outline
1. Introduction
- Traditional teaching of Chemistry is centered mainly on the isolated and fragmented learning of the different tasks and tools of this science, and leaves to one side the development of activities that make it possible to unite skills and knowledge in order to apply them to solving problems. During the last years, the main objective in the teaching of Chemistry has been to develop higher-order cognitive skills (HOCS) in students, including critical thinking, decision making, problem solving (cases) and laboratory practice. [1] Zoller stated that teaching might be improved with teaching strategies that are appropriate and oriented to boosting and strengthening HOCS in students. [2]Thus, an interdisciplinary approach provides an ideal platform for this objective, as it allows the integration of concepts, theories and methods of different study areas, with a focus on the solution of a common problem or on the study of a specific subject. The development of projects with these characteristics favours significant learning in the students. [3]
2. Background: Interdisciplinary in the Teaching of Chemistry
- Several didactic proposals have been made to integrate knowledge of Chemistry in an interdisciplinary education. Results have been promising as students acquire a more holistic awareness and an integral education, placing Chemistry in a real context that relates it to the emerging needs of the present-day world.Environmental Chemistry, for example, is a fertile field for the application of an interdisciplinary strategy. The design and application of educational strategies that use analytical tools to diagnose environmental problems such as water quality, soil degradation or air quality in a region have been reported. The experience generated in the study was highly positive, as the development of an environmental diagnosis prompted interest in the students, which saw the convenience on considering diverse study areas to obtain a particular result. [4] Fundamental aspects of Organic Chemistry have been considered in order to associate related areas to develop complex thought and generate research skills. Central topics in this area of Chemistry were selected, with a focus on nutraceutics i. e. food items or parts of food items that, apart from their nutritional value, provide beneficial effects to health as, for example, antioxidants. The study of Organic Chemistry focused on substances that receive general attention because their properties are beneficial to humans puts diverse aspects into context, which would otherwise be abstract, and enhances research. [5]The interest in developing interdisciplinary strategies is more evident in frontier areas such as nanoscience and nanotechnology, where research is directed to developing new materials and their applications, and necessarily requires dealing with the point of view of different scientific disciplines. [6]We consider that the integral formation of a Chemist requires the development of skills in the laboratory, Therefore it is prioritary to enhance the importance of practical activities, placing them in laboratories designed for different study areas where aspects of two or more theoretical disciplines can be worked on and the students can obtain a more enriching laboratory experience. [7-8]In this paper we reported one teaching strategy focused on interdisciplinary development of chemical courses, this project was made as part of innovaCesal network. [9] The strategy is based on the development of a central topic: acid-base indicators, from the point of view of three courses included in the curriculum of the Undergraduate Program in Chemistry (UC) at Universidad Juárez Autónoma de Tabasco (UJAT): Analytical Chemistry Laboratory 1 (ACL1), Organic Chemistry Laboratory 2 (OCL2) and Chemistry of Natural Products (CNP). The main objective was to develop and enhance higher-order cognitive skills (HOCS) in the students that took part in the project.
3. Methodology
- In order to define an ideal core topic, the teachers responsible of the courses analysed and detected the points of convergence between the different areas. A curricular description of the subjects included in the study is presented in table 1, with a reference to the curricular location and the objectives of each course.
|
4. Result and Discussion
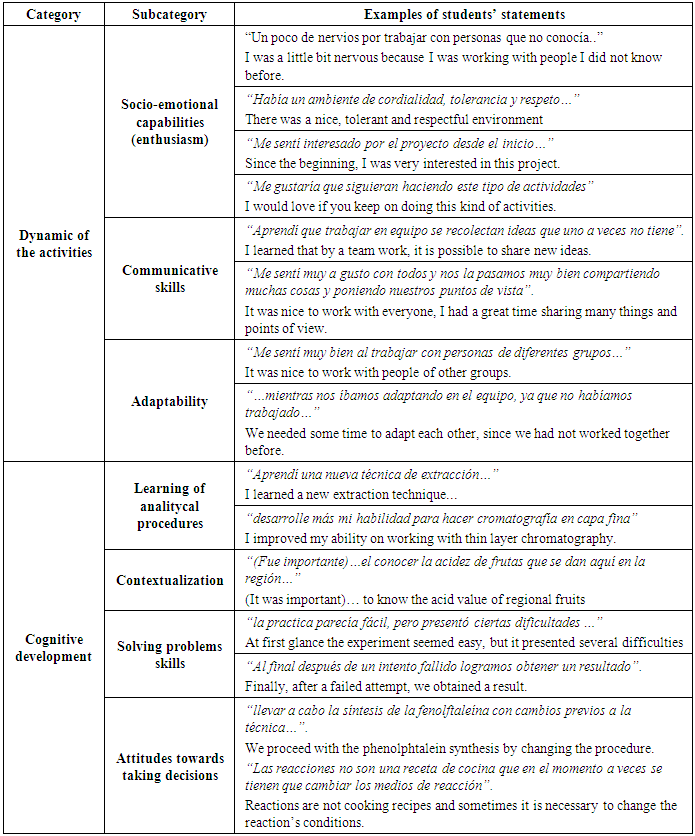
- Three working teams were formed, each of which had one CNP student, one ACL1 student and four OCL2 students. The CNP students (mentors) were responsible for their groups, as they were the most advanced students. Once the teams were formed, activities were proposed in a way that, depending on the subject taken at the time, specific activities were carried out around the acid-base indicator topic. Follow-up integrating activities and analyses of the work were also carried out. A model of the activities followed by each team is synthesized in table 2.
|
|
|
5. Conclusions
- The application of this multidisciplinary work strategy, dealing with a central topic in Chemistry from the perspective of different areas, strengthened collaboration among teachers and made it possible for the students to learn in a significant way, to favour collaborative learning and to generate an holistic view of tasks in Chemistry.The feedback obtained from both the working teams and the teachers who took part in the project proved the accomplishment of HOCS such as critical thinking, problem solving and laboratory practice, as well as the students’ collaborative learning. The experience of sharing in a social network what was learned in the laboratory generated the possibility of carrying out groupal discussion and analyses of specific topics of each of the interdisciplinary projects developed by the students.The results obtained in this study are encouraging and favour proposing a greater number of inter- and multi-disciplinary work strategies for the teaching of Chemistry.
ACKNOWLEDGEMENTS
- The authors thank Innova Cesal for encouraging the project, as well as the “Programa de Fortalecimiento a la Investigación” of the Universidad Juárez Autónoma de Tabasco for financing via the project UJAT-2013-IB-13.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML