-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
2015; 3(3): 44-52
doi:10.5923/j.jlce.20150303.02
Study of Sunscreen Lotions, a Modular Chemistry Project
D. González-Arjona , G. López-Pérez , M. M. Domínguez , S. Cuesta Van Looken
Department Physical Chemistry, Faculty of Chemistry, Seville, Spain
Correspondence to: D. González-Arjona , Department Physical Chemistry, Faculty of Chemistry, Seville, Spain.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
The project described in this paper illustrates the spectrophotometric UV behavior of some representative chemicals used as sun blockers and some over the counter (OTC) sunscreen lotions labeled with different sun protection factor (SPF). The experiments have been carried out in three different solvents, Cyclohexane, Methanol and Dimethyl sulfoxide, which illustrate the solvent effect in the UV spectra. Indices of UV protection have been estimated from the ratios of the areas per unit of wavelength for each UV region (UVA-I, UVA and UVB). Higher indices of UV protection are obtained for higher SPF. The broad spectrum protection (UVA protection) has also been estimated from the critical wavelength value. All OTC sunscreen lotions studied comply with broad spectrum labeling, λ(crit.) ≥370 nm, while the sun blockers studied cannot be classified as such. When comparing the UV spectra for sunscreen lotions that have aged for one year with freshly produced sunscreen, a shift toward the UVB region for aged lotion can be observed. UVA/UVB ratio is the parameter of choice to describe the aging effect, while λ(crit.) is practically insensitive to aging. Sunscreen aging is accompanied by UVA protection failure and therefore renewing sunscreens each season is to be recommended. This project has been carried out for final year students in Chemistry and Pharmacy at different degrees of difficulty.
Keywords: Sunscreens, Graduate Lab, UV spectroscopy
Cite this paper: D. González-Arjona , G. López-Pérez , M. M. Domínguez , S. Cuesta Van Looken , Study of Sunscreen Lotions, a Modular Chemistry Project, Journal of Laboratory Chemical Education, Vol. 3 No. 3, 2015, pp. 44-52. doi: 10.5923/j.jlce.20150303.02.
Article Outline
1. Introduction
- Outdoor activities inevitably result in varying degrees of exposure to sun light. Tanned skin tends to be considered as an outward sign of success and prosperity. Moreover, sun light is well known to have some beneficial effects on health, for example in the bloodstream it increases the number of red blood cells and haemoglobin, as well as facilitates vitamin D3 synthesis through the activation of 7-dehydrocholesterol found in the epidermis [1]. However, excessive exposure is also responsible for skin damage, such as sunburns, aging, skin cancer, adverse effects on the immune system, etc. [2].Fortunately, the human skin has a natural protection mechanism against solar radiation in the form of skin pigmentation via the formation of melanin. The UV radiation in sun light is mainly responsible for the immediate and persistent skin darkening stimulating the melanocytes, which produce melanin, the dark skin pigmentation. Melanin prevents the mutagenesis of cellular DNA by blocking absorption of UV radiation. Nevertheless, the degree of natural protection against UV damage depends on the skin photo-type and age. Children and young adolescents tend to be more vulnerable to UV radiation.Solar radiation covers the wavelength range from 150 nm (Ultraviolet) to 4000 nm (Infrared) in the electromagnetic spectrum. The highest intensity falls in the range of visible light. UV radiation can be divided into UVC (200–290 nm), UVB (290–320 nm) and UVA radiation (320–400 nm). Only part of solar UV radiation reaches the Earth’s surface, the intensity level is lower for shorter wavelengths and depends on the location, season, clouds and level of air pollution. UVC does not reach the Earth’s surface as it is completely absorbed by the atmosphere. UVB radiation also is mainly absorbed by oxygen and ozone, thus the depletion of the ozone layer lowers the degree of absorption. The UVA constitutes 95% of UV radiation that reaches the Earth’s surface. Furthermore, the penetration of solar radiation into the skin has an inverse relationship with the energy of the radiation, thus UVA penetrates deeper into the skin [3].From the earliest times people have been using clothing, umbrellas and hats to avoid the solar radiation’s adverse effects. However, the use of chemicals for sun protection was only first documented in the 20th century [4].Thus, the protective chemicals, sun blockers, are typically applied to the skin as a lotion providing an external armor against the invisible UV radiation [5]. The protection mechanism is similar to that of natural solar protection, i.e. UV light absorption by molecules. The influence of substituent, solvent or pH on the wavelength of maximum absorption of chemical absorbers can be analysed based on simple molecular orbital theory. This absorption is linked to the energy gap between the frontier orbitals, occupied and virtual (HOMO and LUMO) [6].Sun blocking chemicals may be classified according to the kind of protection they offer, either as physical blockers or as chemical absorbers.a) Inorganic (physical blockers).The best-known inorganic UV filters are TiO2 and ZnO particles. However, TiO2 is the most commonly used in the UV filters. A maximum concentration of 25% in a UV filter is recommended for each. The known mechanism of physical sun blocking is through light reflection and scattering, but the small (Nano) particles can absorb part of the UV light [7].b) Organic (chemical absorbers).Organic UV filters include different substance classes (functional groups), which can be classified as UVA and/or UVB filters according to their specific absorption characteristics. They are usually aromatic compounds conjugated with double C=C bonds, having the electronic excitation energy in the UV range. The absorption range and their strength are affected by the position and type of substituents [6, 8].● p-Aminobenzoate derivatives.
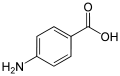 This group of derivatives was one of the first marketed as UV filters. Since 2008, most of them have been banned in the EU.● Benzophenone derivatives.
This group of derivatives was one of the first marketed as UV filters. Since 2008, most of them have been banned in the EU.● Benzophenone derivatives.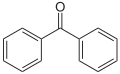 This large family of derivatives shows a very good photo-stability and can be classified as broadband protection over UVB and UVA ranges.● Camphor derivatives.
This large family of derivatives shows a very good photo-stability and can be classified as broadband protection over UVB and UVA ranges.● Camphor derivatives.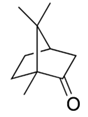 High photostability is a distinguishing feature of this group of derivatives. This property accounts for the fact that they are present in nearly one-third of commercial sunscreens.● Dibenzoylmethane derivatives.
High photostability is a distinguishing feature of this group of derivatives. This property accounts for the fact that they are present in nearly one-third of commercial sunscreens.● Dibenzoylmethane derivatives.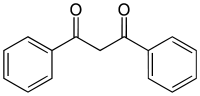 These derivatives are considered as good for UVA protection, but show some degradation in presence of sun light.● Cinnamate derivatives.
These derivatives are considered as good for UVA protection, but show some degradation in presence of sun light.● Cinnamate derivatives.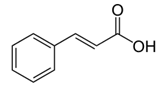 In spite of some photochemical instability, this group of derivatives is one of the most widely used in the manufacture of UV filters. In combination with other UV filters, high SPF values can be achieved.● Salicylate derivatives.
In spite of some photochemical instability, this group of derivatives is one of the most widely used in the manufacture of UV filters. In combination with other UV filters, high SPF values can be achieved.● Salicylate derivatives.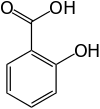 These derivatives show good water resistance and low skin penetration, there are active in the UVB range.● N-Heterocyclic derivarives (imidazol, triazin, benzotriazol …).
These derivatives show good water resistance and low skin penetration, there are active in the UVB range.● N-Heterocyclic derivarives (imidazol, triazin, benzotriazol …).  In order to maximize the effects of topically applied therapeutic drugs, the use of less than 500 Da molecular weight compounds is recommended, known as rule 500 Da [9]. This rule is now no longer strictly applied as new sunscreens are being developed with greater than 500 Da molecular weight. In this way, the absorption through the skin is avoided, minimizing the side effects.The extension and abundance of the chromophores in these compounds provides protection across the entire UVB and UVA ranges.These chemicals play an important protective role in skin health, and therefore their degree of effectiveness has to be controlled. The efficacy of sunscreen products for rating UVB (delayed sunburn) is primarily measured by the so-called sun protection factor, SPF, [10-12] which is the ratio between the minimum erythematic dose (MED) with sun block applied and the MED without sun block:
In order to maximize the effects of topically applied therapeutic drugs, the use of less than 500 Da molecular weight compounds is recommended, known as rule 500 Da [9]. This rule is now no longer strictly applied as new sunscreens are being developed with greater than 500 Da molecular weight. In this way, the absorption through the skin is avoided, minimizing the side effects.The extension and abundance of the chromophores in these compounds provides protection across the entire UVB and UVA ranges.These chemicals play an important protective role in skin health, and therefore their degree of effectiveness has to be controlled. The efficacy of sunscreen products for rating UVB (delayed sunburn) is primarily measured by the so-called sun protection factor, SPF, [10-12] which is the ratio between the minimum erythematic dose (MED) with sun block applied and the MED without sun block: | (1) |
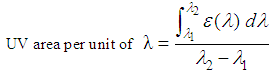 | (2) |
 | Figure 1. Picture of three glass plates covered with different SPF sunscreens illuminated with a black light from a counterfeit banknotes detector |
2. Objectives
- The use and application of UV-Vis spectroscopy is demonstrated by applying it to commonly available products like sunscreens. The importance of the dispersion medium is illustrated by using solvents that differ in polarity, permitivity and H-bond strength [23]. The observed solvatochromic effect forms a starting point for the discussion on how different solvents influence ground and excited states [24]. By using simple spreadsheet calculations, students can estimate and compare the “in vitro” protection efficiency for different chemicals employed. Moreover, the aging effect in the UV protection on the OTC lotions can be studied on the same brand of lotion by using products from previous seasons that have already expired.
3. Experimental
- The experimental study has been carried out using chemicals, which are representative of UV blockers with different functional groups and using sunscreens obtained directly OTC with different SPF.The chemicals investigated have been obtained from Sigma Aldrich [25]: ● 2-Hydroxy-4-methoxybenzophenone, CAS# 131-57-7, Benzophenone-3, Oxybenzone.● 2-Ethylhexyl 4-methoxycinnamate, CAS# 5466-77-3, Octinoxate. ● 2-Ethylhexyl 2-cyano-3, 3-diphenylacrylate, CAS# 6197-30-4, Octocrylene.● 2-Ethylhexyl 4-(dimethylamino)benzoate, CAS# 21245-02-3, Padimate OCommercial sunscreens:Lancaster Tan Deepener Dry Oil, SPF10 and SPF 15. Active UV components [26]:● Bis-Ethylhexyloxyphenol Methoxyphenyl Triazine (CAS# 187393-00-6)● Butyl Methoxydibenzoylmethane (CAS # 70356-09-1)● Methoxycinnamate (CAS# 5466-77-3)● Octocrylene (CAS# 6197-30-4)Eucerin Daily Protection Face Lotion SPF 30. Active UV components [27]:● Ensulizole (CAS# 27503-81-7)● Octinoxate (CAS# 5466-77-3)● Octocrylene (CAS#, 6197-30-4)● Zinc Oxide (CAS# 1314-13-2)● Titanium Dioxide (CAS# 13463-67-7)Avène Stick 50+, one stick from the current season and other from the previous one. Active UV components [28]:● Butyl Methoxydibenzoylmethane (CAS # 70356-09-1)● Octocrylene (CAS# 6197-30-4)● Bis-Ethylhexyloxyphenol Methoxyphenyl Triazine (CAS# 187393-00-6)● Titanium Dioxide (CAS# 13463-67-7)Three different solvents, analytical reagent grade, have been selected, one non-polar, Cyclohexane (Cx), and two having a polar character, one protic, Methanol (MetOH) and Dimethyl sulphoxyde (DMSO) as aprotic. The solvents were stored in amber bottles with a 4Å molecular sieve as dessicant.The procedure for the solubilization of some samples was improved by using an ultrasound bath, product of Selecta [29]. The sunscreens with high degree of solar protection can include in their formulation TiO2 and ZnO solid particles as inorganic sun blockers. Solutions having particles in suspension were filtered through a 0.45 μm Nylon filter to obtain clear working solutions.In order to get absorbance values in the optimal range (0.5–1.5), a 100mL of stock solution approx. 10-3 mol/L for each compound and solvent were prepared and stored in amber bottles. From them, working diluted solutions (approx. 10-5 mol/L) were prepared to fulfill the optimal absorbance range in the corresponding solvent. In the case of sunscreens, the same absorbance criterion was observed, but the solution concentrations were expressed in g/L.Spectrophotometric measurements were performed with a Shimadzu UV Spectrophotometer Mini-1240 controlled by UVProbe version 2.31 software. A digital UV spectrum can be easily stored on any common spreadsheet saved from a text file. If the spectrophotometer only generates the spectrum as a hard copy graphical output, it is possible to obtain the spectrum in digital format by scanning the spectrum image by using freely available digitization software like WinDig [30].Hellma UV range Quartz Suprasil [31] precision spectrophotometric cells, with 10mm path length, were employed.
4. Results and Discussion
- Experimental UV spectra were recorded from 250 nm to 400 nm every 0.5 nm for each compound in the three solvents. These spectra in digital format were stored on a spreadsheet, one for each compound. Using a spreadsheet template, included in the supplemental material, the spectra are transformed to allow a better comparison with the plot of molar extinction coefficient, ε, vs. wavelength,
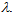 In this way, a direct comparison of the absorption capacity can be done independent of the actual sun blocker or sunscreen compound concentration. Figure 2 shows the spectra as ε vs.
In this way, a direct comparison of the absorption capacity can be done independent of the actual sun blocker or sunscreen compound concentration. Figure 2 shows the spectra as ε vs. 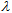 plots, for the Padimate O, CAS# 21245-02-3. The UVB (290–320nm) and UVA (290–400nm) regions are shaded in the plot.
plots, for the Padimate O, CAS# 21245-02-3. The UVB (290–320nm) and UVA (290–400nm) regions are shaded in the plot.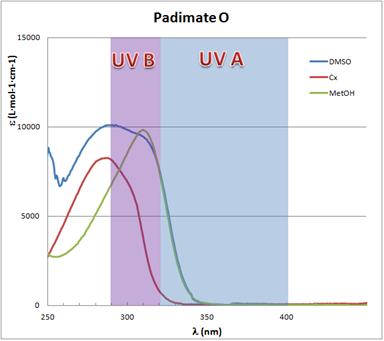 | Figure 2. Padimate O UV-Vis spectra in different solvents |
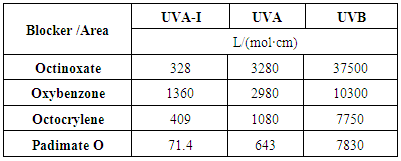
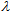 in a UV wavelength range (per unit wavelength), provides a quantitative protection index against the UV radiation in the spectral zone, eqn. (2).It is important to note that a constant amount of 2 mg per square centimeter of substrate [10] is applied, in agreement with FDA recommendations for in vitro testing of externally applied drugs. Thus, the area under the plot, ε vs.
in a UV wavelength range (per unit wavelength), provides a quantitative protection index against the UV radiation in the spectral zone, eqn. (2).It is important to note that a constant amount of 2 mg per square centimeter of substrate [10] is applied, in agreement with FDA recommendations for in vitro testing of externally applied drugs. Thus, the area under the plot, ε vs. 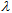 should be correlated with the UV protection in vitro. In fact, this protection index is close to that reported for in vitro testing in accordance with the FDA recommended procedure, since in both cases the estimated area is proportional to the mass or molar extinction coefficient. Figure 3 shows a sample spreadsheet which reports the Octocrylene areas obtained for the three solvents in L/(mol·cm) and L/(g·cm). Those areas have been easily estimated on the spreadsheet using the trapezoidal rule. The trapezoid bases are the ε(i) values, and the heights are
should be correlated with the UV protection in vitro. In fact, this protection index is close to that reported for in vitro testing in accordance with the FDA recommended procedure, since in both cases the estimated area is proportional to the mass or molar extinction coefficient. Figure 3 shows a sample spreadsheet which reports the Octocrylene areas obtained for the three solvents in L/(mol·cm) and L/(g·cm). Those areas have been easily estimated on the spreadsheet using the trapezoidal rule. The trapezoid bases are the ε(i) values, and the heights are 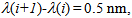 so each trapezoid area is:
so each trapezoid area is: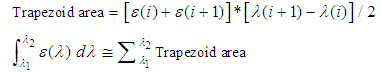 | (3) |
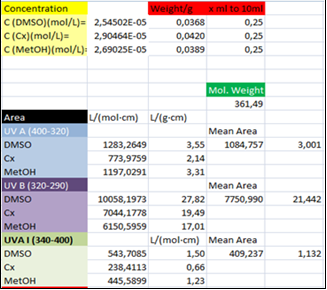 | Figure 3. Part of the Octocrylene spreadsheet snapshot displaying the calculated area under UV zones |
 plots. Padimate O spectra for both polar solvents are shifted to longer wavelengths, i.e. a bathochromic shift.This is an indication of the different interaction with the solvent in the UV absorption process, that is, the solvatochromic effect. Thus, the energy in the electronic transition from ground state to the excited state is lower for polar solvents. In this case, the bathochromic shift implies that the excited state has a stronger polar character compared with the ground state, causing it to be stabilized in a polar environment [24]. Nevertheless, for other compounds the effect is absent as illustrated by the Oxybenzone spectra of Figure 4.
plots. Padimate O spectra for both polar solvents are shifted to longer wavelengths, i.e. a bathochromic shift.This is an indication of the different interaction with the solvent in the UV absorption process, that is, the solvatochromic effect. Thus, the energy in the electronic transition from ground state to the excited state is lower for polar solvents. In this case, the bathochromic shift implies that the excited state has a stronger polar character compared with the ground state, causing it to be stabilized in a polar environment [24]. Nevertheless, for other compounds the effect is absent as illustrated by the Oxybenzone spectra of Figure 4.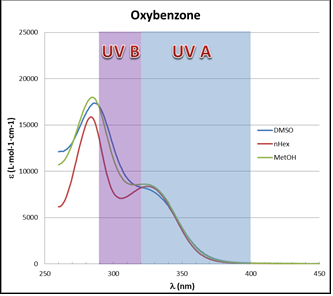 | Figure 4. Oxybenzone UV-Vis spectra in different solvents |
|
|
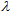 (in nm) plots for Eucerin SPF 30 have a marked contribution from the UVA spectral zone as is desirable for a broad spectrum sunscreen. Different values for the extinction coefficient can be observed for the different solvents with some solvatochromic effect.
(in nm) plots for Eucerin SPF 30 have a marked contribution from the UVA spectral zone as is desirable for a broad spectrum sunscreen. Different values for the extinction coefficient can be observed for the different solvents with some solvatochromic effect.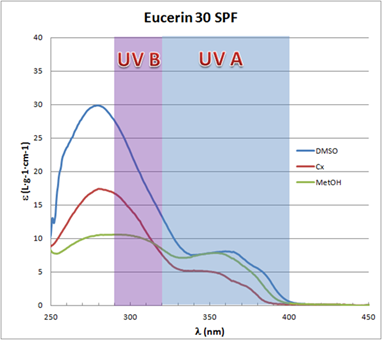 | Figure 5. Eucerin SPF 30 UV-Vis spectra in different solvents |
|
|
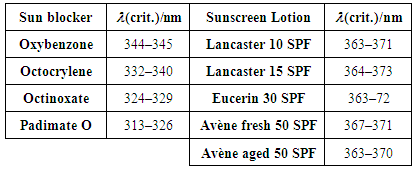
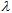 (crit.), up to which 90% of the cumulative area under the full UV spectrum (290–400 nm) is covered. A large critical wavelength is indicative of better UV protection. A product with a
(crit.), up to which 90% of the cumulative area under the full UV spectrum (290–400 nm) is covered. A large critical wavelength is indicative of better UV protection. A product with a 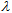 (crit.) ≥ 370 nm is considered to have broad spectrum protection. This parameter can be easily estimated from ε vs.
(crit.) ≥ 370 nm is considered to have broad spectrum protection. This parameter can be easily estimated from ε vs. 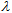 plots stored as spreadsheets. Table 5 shows that
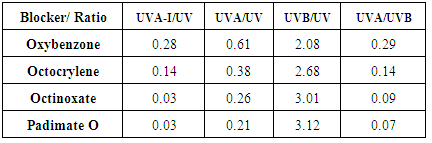
plots stored as spreadsheets. Table 5 shows that 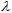 (crit.) values for the sun blockers studied are between 315 nm and 345 nm. These low UV protection
(crit.) values for the sun blockers studied are between 315 nm and 345 nm. These low UV protection 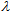 (crit.) values are in agreement with low UVA-I mean areas and UVA-I/UV ratios for these compounds. However,
(crit.) values are in agreement with low UVA-I mean areas and UVA-I/UV ratios for these compounds. However, 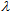 (crit.) values for all sunscreen lotions are consistent with their classification as providing broad spectrum protection. Moreover, their high values seem to be independent of the fact that inorganic sun blocker for sunscreen solutions with high SPF have been removed.
(crit.) values for all sunscreen lotions are consistent with their classification as providing broad spectrum protection. Moreover, their high values seem to be independent of the fact that inorganic sun blocker for sunscreen solutions with high SPF have been removed.
|
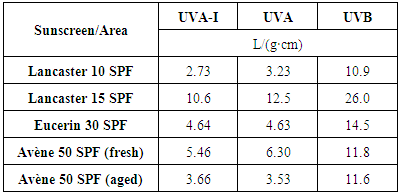
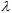 plots in different solvents for the fresh and aged product, respectively. These plots show that the ε in the UVA zone is lower for the aged product. The opposite occurs in the UVB zone, where the ε values are larger for the aged product. The data for Avène lotion collected in Tables 3 and 4 quantify these observations.
plots in different solvents for the fresh and aged product, respectively. These plots show that the ε in the UVA zone is lower for the aged product. The opposite occurs in the UVB zone, where the ε values are larger for the aged product. The data for Avène lotion collected in Tables 3 and 4 quantify these observations.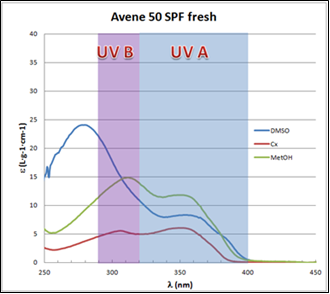 | Figure 6. Avène SPF 50 fresh UV-Vis spectra in different solvents |
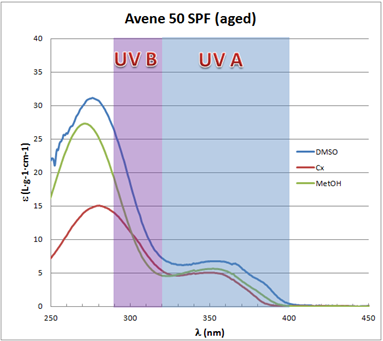 | Figure 7. Avène SPF 50 aged UV-Vis spectra in different solvents |
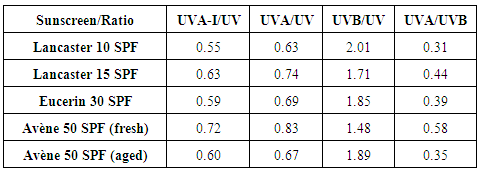
 (crit.) values to aging, see Table 5. Figures 6 and 7 show that the greater increase in absorptive strength for the aged product takes place at wavelengths less than 290 nm, which are not taken into account in the
(crit.) values to aging, see Table 5. Figures 6 and 7 show that the greater increase in absorptive strength for the aged product takes place at wavelengths less than 290 nm, which are not taken into account in the 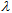 (crit.) estimation of UV protection. Taking into account that UV ratios are calculated as ratios of arithmetic means, and that the UVB contribution is always greater than that of UVA, the UVA/UVB ratio will be more sensitive to aging than the UVA/UV ratio, see eqns. (4).
(crit.) estimation of UV protection. Taking into account that UV ratios are calculated as ratios of arithmetic means, and that the UVB contribution is always greater than that of UVA, the UVA/UVB ratio will be more sensitive to aging than the UVA/UV ratio, see eqns. (4).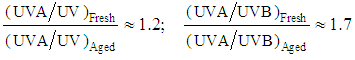 | (4) |
5. Conclusions
- This experimental study is proposed as a final year project for students of Chemistry and/or Pharmacy. Readily available lab equipment that is commonly employed, the relative ease with which these experiments can be carried out, together with the use of OTC products for everyday use, would make it a very attractive project for students.The mean UV area by wavelength and the UV ratios are suitable parameters, which qualitatively and semi-quantitatively describe the UV blocking effect for both sun blockers’ active components as well as OTC sunscreen products. It is worthwhile to take into account the influence of the solvent and/or excipients on the UV-Vis shift, as some products lose some of their UVA protection capacity in polar solvents. The procedure can be employed to compare fresh and aged OTC products, being the UVA/UVB mean area ratio the parameter of choice to characterize protection levels. The aging effect is more important in the UVA region. Thus, the use of freshly prepared sunscreens rather than reuse of aged stock from the previous season is recommended.
ACKNOWLEDGEMENTS
- The authors are very grateful to Prof. W.H. Mulder for his helpful comments and suggestions. We also thank Undergraduate Chemistry students A. Martínez Pascual and A. Palacios Morillo, for their participation in developing part of this project.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML