-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
2014; 2(5): 85-89
doi:10.5923/j.jlce.20140205.02
Polymerization of Hot Melt Adhesives from Dicarboxylic Fatty Acid for Introductory Organic Chemistry Laboratories
Nayef Ghasem
Department of Chemical and Petroleum Engineering, UAE University, Alain city, UAE
Correspondence to: Nayef Ghasem, Department of Chemical and Petroleum Engineering, UAE University, Alain city, UAE.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Synthesis of hot melt adhesives from dicarboxylic fatty acid and ethylenediamine is presented for the undergraduate organic laboratory. The project fulfills the requirement of undergraduate chemistry and chemical engineering students. The experimental setup is utilized for the determination of polymerization reaction rate constants and reaction order. The apparatus also provides a safe method for observing polymer formation. In this experiment students prepare, react, and convert dicarboxylic fatty acid to hot melt adhesive. Students will find reaction order and specific reaction rate of stoichiometric proportions of ethylenediamine and dicarboxylic fatty acid to produce polyamide adhesives using batch reactor. The experiment will expose students to observe the steps of adhesive formation along with problems phasing polycondensation reactions due to foam formation.
Keywords: Curriculum, Second-Year Undergraduate, Laboratory Instruction, Organic Chemistry, Polymerization, Kinetic, Synthesis
Cite this paper: Nayef Ghasem, Polymerization of Hot Melt Adhesives from Dicarboxylic Fatty Acid for Introductory Organic Chemistry Laboratories, Journal of Laboratory Chemical Education, Vol. 2 No. 5, 2014, pp. 85-89. doi: 10.5923/j.jlce.20140205.02.
1. Introduction
- The main objective of engineering education is to prepare students to practice engineering. The emphasis on laboratories has varied over the years. While much attention has been paid to curriculum and teaching methods, relatively little has been written about laboratory instruction [1, 2]. A one-term synthesis experimentation on the synthesis of nylon 6,6 was presented for the undergraduate organic laboratory by Dintzner et al. [3]. In the experiment students react, recycle and convert cyclohexanol to nylon 6,6. The task includes the principles of green chemistry. In the present work, dicarboxylic fatty acids is employed to synthesize and formulate hot melt adhesives. The polymerization reaction between dicarboxylic fatty acid (HOOCC34H68COOH) and ethylenediamine (H2NCH2CH2NH2) is as follows [4-6]:
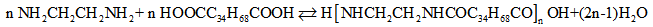 In the fatty polyamide industry, the water produced during the reaction is purged out to minimize the reverse reaction. Besides, the evaporation of ethylenediamine during the reaction is another important factor that needs to be considered. If the evaporation becomes too great, the loss of ethylenediamine will cause an imbalance in the acid and amine values and this will affect the final products. The kinetics of the reaction between ethylenediamine and dicarboxylic fatty acids in the melt phase within the temperature range 210–250℃ has been experimentally investigated. The produced water vapor and vaporized ethylenediamine was purged by nitrogen bubbling, the anaylsis revealed that the reaction was second order with activation energy of 76.44 kJ/mole for conversions up to 90%. For higher conversions, the reaction was third order overall with activation energy of 68.88 kJ/mol [7-10]. Another work carried out on the kinetics of the reaction between C36 dicarboxylic fatty acids and diethylenetriamine and triethylenetetramine in the temperatures range 147–192℃, disclosed that the reactions followed an overall second order kinetics and had activation energies of 60.8 and 51.7 kJ/mol, respectively [11]. These polyamides are known as reactive polyamides because they can be cross linked with other resins such as epoxy resins and they are not linear. Kinetic studies on the reaction between ethylenediamine and C36 dicarboxylic fatty acids using benzyl alcohol as a solvent was investigated. The reaction was performed in the temperature range 162–192℃ and the kinetics was determined from the change in acid values. The reaction was found to be third order overall and had activation energy of 128.9 kJ/mol [8]. The order of the reaction at conversions above 90% changed from 2nd to 3rd order. This phenomenon has been reported for many polycondensation polymerizations reactions such as with polyesters [12, 13]. The change in the order of reaction for fatty polyamides has been verified and its effect on the reverse reaction is evaluated. The reverse reaction at the end of the reaction is important in order to ascertain whether there is a need to apply a vacuum or nitrogen bubbling to complete the reaction. Nitrogen bubbling inside the reaction mass has been used and this causes the irreversibility of the reaction, where this differs from the work that the nitrogen was introduced from the top of the reactor and used to sweep the vapors (mainly water vapor and evaporated ethylenediamine) on the surface of the reaction mass. The kinetics of reaction up to 90% conversion was modeled [14]. In the present experiment, vacuum was used to sweep out water vapor and evaporated ethylenediamine.
In the fatty polyamide industry, the water produced during the reaction is purged out to minimize the reverse reaction. Besides, the evaporation of ethylenediamine during the reaction is another important factor that needs to be considered. If the evaporation becomes too great, the loss of ethylenediamine will cause an imbalance in the acid and amine values and this will affect the final products. The kinetics of the reaction between ethylenediamine and dicarboxylic fatty acids in the melt phase within the temperature range 210–250℃ has been experimentally investigated. The produced water vapor and vaporized ethylenediamine was purged by nitrogen bubbling, the anaylsis revealed that the reaction was second order with activation energy of 76.44 kJ/mole for conversions up to 90%. For higher conversions, the reaction was third order overall with activation energy of 68.88 kJ/mol [7-10]. Another work carried out on the kinetics of the reaction between C36 dicarboxylic fatty acids and diethylenetriamine and triethylenetetramine in the temperatures range 147–192℃, disclosed that the reactions followed an overall second order kinetics and had activation energies of 60.8 and 51.7 kJ/mol, respectively [11]. These polyamides are known as reactive polyamides because they can be cross linked with other resins such as epoxy resins and they are not linear. Kinetic studies on the reaction between ethylenediamine and C36 dicarboxylic fatty acids using benzyl alcohol as a solvent was investigated. The reaction was performed in the temperature range 162–192℃ and the kinetics was determined from the change in acid values. The reaction was found to be third order overall and had activation energy of 128.9 kJ/mol [8]. The order of the reaction at conversions above 90% changed from 2nd to 3rd order. This phenomenon has been reported for many polycondensation polymerizations reactions such as with polyesters [12, 13]. The change in the order of reaction for fatty polyamides has been verified and its effect on the reverse reaction is evaluated. The reverse reaction at the end of the reaction is important in order to ascertain whether there is a need to apply a vacuum or nitrogen bubbling to complete the reaction. Nitrogen bubbling inside the reaction mass has been used and this causes the irreversibility of the reaction, where this differs from the work that the nitrogen was introduced from the top of the reactor and used to sweep the vapors (mainly water vapor and evaporated ethylenediamine) on the surface of the reaction mass. The kinetics of reaction up to 90% conversion was modeled [14]. In the present experiment, vacuum was used to sweep out water vapor and evaporated ethylenediamine. 2. Construction
- The polyamidation reaction was carried out in one liter jacketed reactor (Janke & Kunkel IKA-labortechnik, Germany, Figure 1 and 2) equipped with anchor style mixer, sampling port, and thermometer for temperature measurement. Vacuum tube attached with vacuum pump for removal of the generated water vapor. The temperature was maintained by oil bath. The reactor was charged with half mole of fatty acid and equivalent molar amount of ethylenediamine. The water generated by the polymerization reaction was sucked out of the system through the vacuum pump to a condenser attached to a water-cooling bath maintained at 4℃. Removal of water vapor from the system helped the reaction to go in the forward direction. The acid and amine values were determined as per ASTM D-1980-67 and ASTM D-2074-62T, respectively, with a neutral solution (1:1, v/v) of ethanol and toluene for dissolving the samples.
 | Figure 1. Experimental setup of the polyamidation reaction |
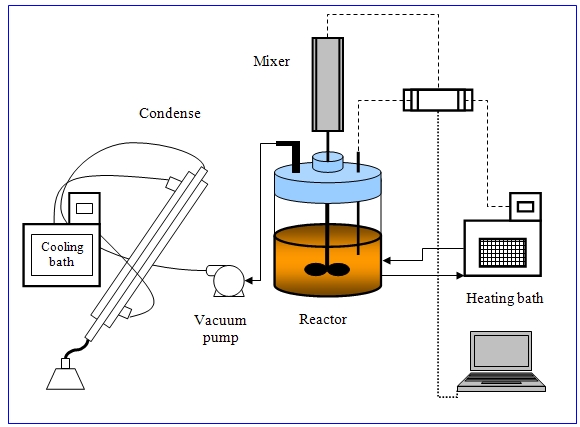 | Figure 2. Schematic diagram of the experimental setup |
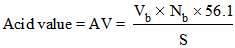 where Vb is the volume in ml of KOH solution required for the titration, Nb is the normality of the KOH solution, and S is the specimen weight in grams. The value 56.1 is the molecular weight of KOH. For the amine values, 5 g of the polyamide sample weighed to 0.1 mg was weighed and transferred into a 250-ml flask, 50 ml of xylene and isopropanol (1/1: v/v) was boiled for 1 min to drive off any free ammonia that may be present, and the boiled mixture was added to the 250 ml flask. The solution was cooled to room temperature and five drops of bromophenol blue indicator was added to the solution and titrated while swirling with 0.2 N HCl to the yellow ends. The total amine value was calculated as follows:
where Vb is the volume in ml of KOH solution required for the titration, Nb is the normality of the KOH solution, and S is the specimen weight in grams. The value 56.1 is the molecular weight of KOH. For the amine values, 5 g of the polyamide sample weighed to 0.1 mg was weighed and transferred into a 250-ml flask, 50 ml of xylene and isopropanol (1/1: v/v) was boiled for 1 min to drive off any free ammonia that may be present, and the boiled mixture was added to the 250 ml flask. The solution was cooled to room temperature and five drops of bromophenol blue indicator was added to the solution and titrated while swirling with 0.2 N HCl to the yellow ends. The total amine value was calculated as follows: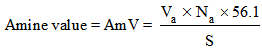 where Va is the volume of HCl solution required for the titration in ml, Na is the normality of the HCl solution, and S is the specimen’s weight in grams. The number average molecular weight, Mn was calculated on the basis of the following equation.
where Va is the volume of HCl solution required for the titration in ml, Na is the normality of the HCl solution, and S is the specimen’s weight in grams. The number average molecular weight, Mn was calculated on the basis of the following equation.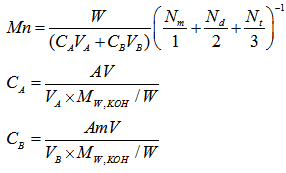 where W is the weight of solid sample titrated;
where W is the weight of solid sample titrated; 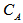 ,
, 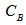 ,
, 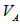 , and
, and 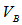 are the concentrations and actual volumes used from the standard solutions of the sodium hydroxide and the hydrochloric acid, respectively.
are the concentrations and actual volumes used from the standard solutions of the sodium hydroxide and the hydrochloric acid, respectively.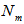 ,
, 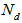 and
and 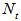 are the percentage concentrations of the monomers with one, two, and three functional groups (acid and amide) in the products. Where
are the percentage concentrations of the monomers with one, two, and three functional groups (acid and amide) in the products. Where 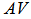 is the acid value, and
is the acid value, and 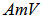 is the amine value,
is the amine value, 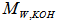 is the molecular weight of potassium hydroxide (MW = 56.1 g/mol). Rearrange the previous equations yields:
is the molecular weight of potassium hydroxide (MW = 56.1 g/mol). Rearrange the previous equations yields: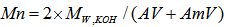 Glass transition temperature, melting points, and heat of fusion were measured using differential scanning Calorimetry (DSC) with heating rate of 20℃/min and under nitrogen atmosphere from room temperature to 200℃. Less than 1 gram of the produced polymer is melted on a glass slide as a film under vacuum for half an hour. The sample was kept at 120℃ in an oven for the removal of water. The sample was then cooled. The melting point of the prepared sample was obtained using DSC.
Glass transition temperature, melting points, and heat of fusion were measured using differential scanning Calorimetry (DSC) with heating rate of 20℃/min and under nitrogen atmosphere from room temperature to 200℃. Less than 1 gram of the produced polymer is melted on a glass slide as a film under vacuum for half an hour. The sample was kept at 120℃ in an oven for the removal of water. The sample was then cooled. The melting point of the prepared sample was obtained using DSC. 3. Data Analysis
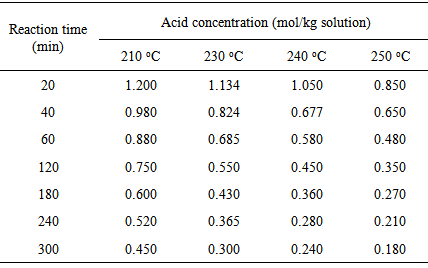
- Methods to demine rate law parameters for homogenous reactions such as differential, integral and least squares method of data analysis is summarized elsewhere [15]. Advanced calculation methods can be performed using Easy-Fit software package [16]. Studies on the kinetics of polyamidation reaction of C36 dicarboxylic fatty acids and ethylenediamine for the synthesis of hot melt adhesives followed overall second-order with activation energy of 18.2 kcal/gmol up to 90% conversion, and overall third-order kinetics with activation energy of 16.583 kcal/mol. In the present experiment, the kinetics of polymerization at different reaction temperatures was investigated under vacuum. Acid concentration at four different reaction temperature (210, 230, 240, 250℃) versus time is shown in Table 1. Figure 3 shows the effect of reaction temperature on the acid concentration with reaction time. Figure 3 reveals that, the acid value decreases with increase in reaction time due to consumption rate of the dicarboxylic fatty acid as reaction proceeds. It can be seen that as reaction temperature increases the rate of the go down in the acid value increases due to the increase in consumption rate of dicarboxylic fatty acid. Assuming that the reaction is irreversible, because of the continuous removal of produced water using the strong vacuum, the following equations can be set to describe the batch reactor. Using the integral method and assuming the reaction is overall second order, the initial concentrations of amine and acid are equal, lead to the following first order differential equation [17].
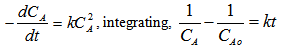 A plot of
A plot of 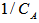 versus
versus 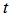 (see Figure 4) will give values of specific reaction rate constant,
(see Figure 4) will give values of specific reaction rate constant, 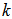 , at different reaction temperatures. Using the Excel software package, values of specific reaction rate constant at different reaction temperatures are shown in Table 2. The data of reaction rate constant
, at different reaction temperatures. Using the Excel software package, values of specific reaction rate constant at different reaction temperatures are shown in Table 2. The data of reaction rate constant 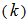 vs. temperature were found to fit the Arrhenius equation,
vs. temperature were found to fit the Arrhenius equation, 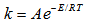 ,
, 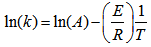
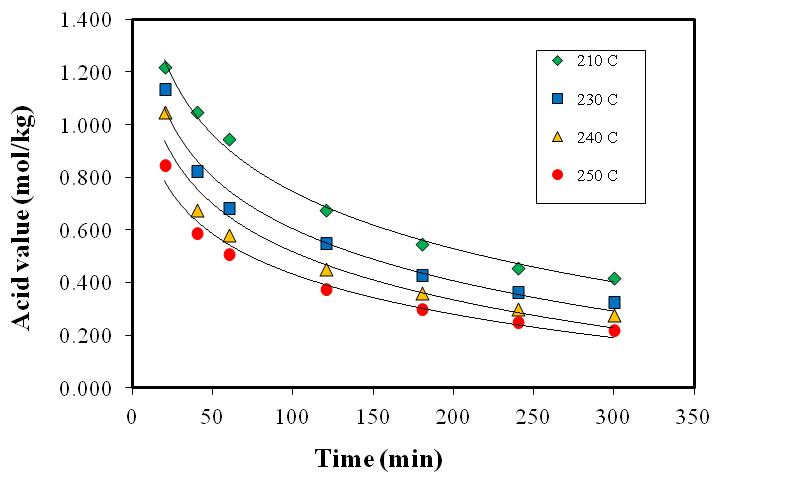 | Figure 3. Acid concentrations various reaction times at different reaction temperatures |
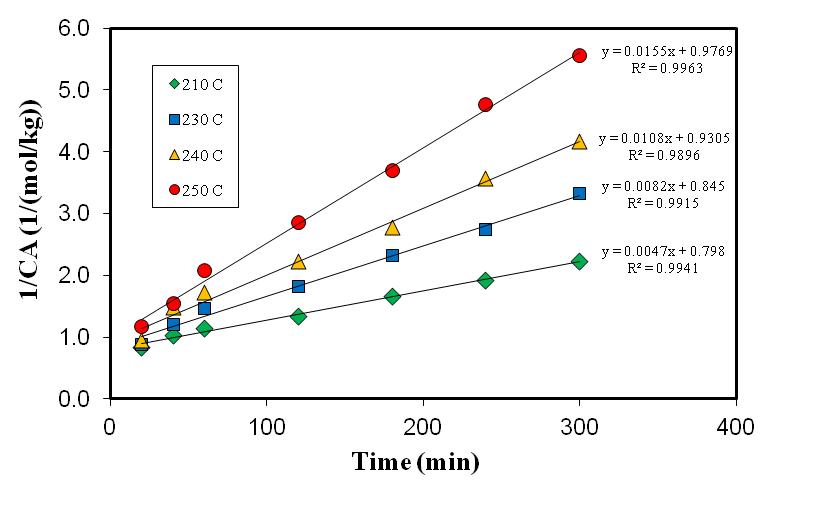 | Figure 4. Reaction rate constant at different reaction temperatures |
|
|
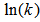 versus
versus 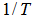 as shown in Figure 5 gives the slope of 7399.2 K:
as shown in Figure 5 gives the slope of 7399.2 K: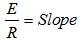 ,
, 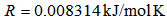 ,
, 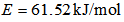
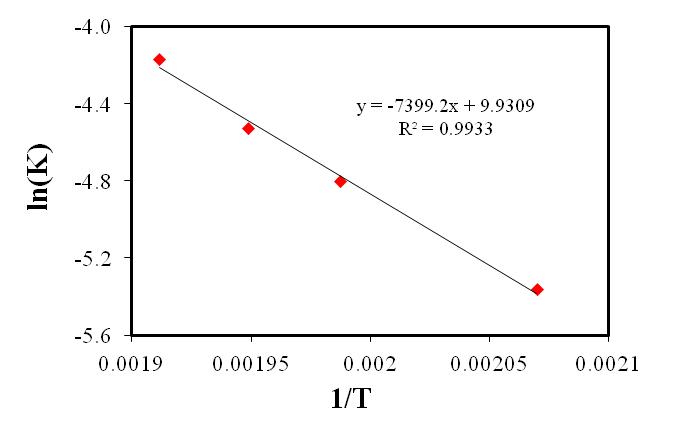 | Figure 5. Forward specific reaction rate constant versus inverse of reaction temperatures |
4. Conclusions
- The introduction of polyamidation reaction between C36 dicarboxylic fatty acid and ethylenediamine for the undergraduate organic laboratories provides students with background concerning the principles of synthesis of adhesives. Students are also gaining hands-on experience with polymerization reaction rate and how to accelerate reaction by adding catalyst such as Ortho-phosphoric acid and avoiding reversible reactions through removing one of the products such as water in this case. The students will be aware on the effects of certain operating conditions such as; effect of vacuum, flashing with nitrogen, and nitrogen bubbling on the polymerization reaction.
5. Safety Precautions
- Lab coat, mask and safety glasses should be worn throughout the experiments. The apparatus should be placed in vacuum hood to get red from vapors and smell. The apparatus should be cleaned while the polymer is hot otherwise it will stick with reactor walls.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML