-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
2014; 2(4): 73-78
doi:10.5923/j.jlce.20140204.04
How Old is My Bronze Cannon? A Laboratory Exercise Linking Analytical Chemistry, Spectroscopy and Metallurgy
David J. Henry, Damian W. Laird
Chemical and Metallurgical Engineering and Chemistry, School of Engineering and IT, Murdoch University, Murdoch, Western Australia, Australia
Correspondence to: David J. Henry, Chemical and Metallurgical Engineering and Chemistry, School of Engineering and IT, Murdoch University, Murdoch, Western Australia, Australia.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Details are provided of a simple, quick, and safe laboratory exercise to analyze the tin content of a bronze sample. The experiment is set in the context of analyzing filings from a bronze artefact (“cannon”) of unknown origin. Students work in teams to complete separate parts of the experiment and to produce replicate sets of results. The procedure is designed in such a way as to demonstrate the basic principles of oxidation and reduction, spectroscopy and analytical chemistry at a level suitable for upper secondary school students. It also provides an opportunity to introduce students to advanced analytical instrumentation that is not generally available in secondary schools. The experiment was developed to be completed in a single 50 minute session and has been successfully conducted with over 200 middle- to upper-level secondary school students.
Keywords: Metals, Oxidation, Analytical Chemistry, Spectroscopy, Interdisciplinary, Multidisciplinary, Public Understanding/Outreach, Hands-On Learning, High School/Introductory Chemistry, Applications of Chemistry
Cite this paper: David J. Henry, Damian W. Laird, How Old is My Bronze Cannon? A Laboratory Exercise Linking Analytical Chemistry, Spectroscopy and Metallurgy, Journal of Laboratory Chemical Education, Vol. 2 No. 4, 2014, pp. 73-78. doi: 10.5923/j.jlce.20140204.04.
Article Outline
1. Introduction
- Recently we reported a selective complexation experiment that was developed to introduce secondary school students to some practical aspects of chemistry that are particularly relevant to Extractive Metallurgy and associated areas. [1] This paper describes a second chemistry focused exercise designed to highlight analysis using instrumentation not available in schools. A key aspect of this experiment is to demonstrate how some of the science that students have already encountered is particularly relevant to a range of applications and industries. In our context it was also important to link this experiment to Minerals and Metallurgy activities such as tin smelting and production of alloys. The activity was designed to challenge participants, rather than simply repeating what students might otherwise be able to do in their current class room. This was achieved by extending on the basic science and chemistry concepts that students would already be aware of and making use of advanced instrumentation (Flame Atomic Absorption Spectroscopy) not generally available to high schools. The exercise was designed to include a safety induction, teamwork, replication of measurements, safe laboratory working practices, and be accessible to students with a range of abilities and laboratory experience (from those doing general science up to students undertaking capstone secondary school chemistry classes). This all needed to be achieved in a single 50 minute session.Accurate quantitative analysis of single metal elements in samples is critically important, not only for the minerals industry but in a range of other fields including environmental monitoring, metallurgical quality control and some pharmaceuticals. Although a range of techniques can be applied for the analysis of metals including colorimetric techniques, UV/V is spectroscopy and electro-analytical methods, one of the most widely used techniques for the determination of metal ion concentrations is Flame Atomic Absorption Spectroscopy (FAAS).The principal components of bronze are copper and tin, with copper as the primary component and tin as the secondary component. The exact composition of bronze objects can vary significantly with origin and age of the object. For example bronze mirrors and weapons from the Chou and Han periods (China, 400 BC – 200 AD) have consistently been found to have 70 – 75 wt% Cu and 20 – 25 wt% Sn and 1 – 5 wt% Pd. [2] A medieval bronze bell excavated from the crypt of the church of Saints Peter and Paul in Brno was found to have a composition of 68.4 wt% copper, 20.0 wt% tin, 3.6 wt% lead, 4.6 wt% antimony, 3.2 wt% arsenic and 0.3 wt% chlorine. [3] In comparison, bronze cannons from 17th century Swedish warships have been found to have very high copper content (~96 wt%) and relatively low tin content (~ 3 wt%). [4] Modern bronzes come in a range of compositions depending on the intended application. Phosphor bronze (~95 wt% Cu, ~4 wt% Sn, and ~ 1 wt% P) is often used in marine environments where good corrosion and wear resistance is required. In comparison, many modern bearings are composed of oil impregnated porous bronzes, with a copper to tin ratio of 90:10. [5] FAAS has been successfully used to determine the concentrations of both the major components as well as many of the minor components in bronze objects including lead in ancient coins, [6] phosphorus in bronze artefacts [7] and products from the corrosion of Admiralty brass in seawater. [8] In this report we present a simple experiment to describe the basic principles of emission and absorption spectroscopy and their use to quantify one of the components (tin) of a bronze sample.
2. Experimental
2.1. Materials and Equipment
- Flame testMeker BurnerSingle metal ion solutions (all 1 M) placed in spray bottles for the first part of the flame test: NaNO3, KNO3, Ca(NO3)2, Sr(NO3)2, Ba(NO3)2, Cu(NO3)2, [Cu(MAC)](ClO4)2 MAC = trans [14] diene or5,7,7,12,14,14-hexamethyl-1,4,8,11-tetra- azacyclotetradeca-4,11-diene, SnCl2.For the second part of the flame test a selection of binary metal ion solutions were used in spray bottles as follows: 0.5 M KNO3 / 0.5 M Sr(NO3)2, 0.5 M NaNO3 / 0.5 M Ba(NO3)2, 0.5 M Ca(NO3)2 / 0.5 M Cu(NO3)2, 0.5 M Cu(NO3)2 / 0.5 M SnCl2.Bronze analysisVarian SpectrAA 50 Atomic Absorption SpectrometerPhotorn Sn Hollow Cathode Lamp (λ = 235.5 nm)1000 ppm (1000 mg L-1) tin stock solution, 12 M hydrochloric acid (5 mL), 14 M nitric acid (10 mL), The bronze samples used in our analysis were obtained from phosphor bronze rod which typically has a tin content of 4 wt%.
2.2. Scenario
- We proposed a scenario to the students that they had been contracted by a museum to determine the tin content of a bronze sample taken from an artefact (a cannon) as a first step to verifying the age of the object. The discussion revolves around the fact that bronze objects from different eras and locations have subtly different compositions. Although tin content is not as useful as lead content for determination of the age of a bronze object, it has a number of educational and safety advantages over lead. The focus of this exercise is to introduce students to some simple analytical chemistry and the application of spectroscopy. One of the goals of this experiment is to show that an analytical instrument can provide a “number” for a sample but that this number is only useful when placed in the context of known values for that particular characteristic. This was achieved through a brief discussion of the concept of a calibration curve obtained from accurately prepared standard solutions and the need for care and accuracy in preparing an unknown sample for analysis.
2.3. Execution
- Following this the group is divided into teams responsible for either preparing sets of standard solutions or, alternatively, to digest the bronze samples and carefully prepare these for analysis.The team preparing the calibration standards (up to 8 students) was further divided into two groups, each of 2 – 4 students, to prepare duplicate sets of standards from the stock solution. To accommodate different levels of laboratory skills and the time limitations, the solutions were prepared using adjustable pipettes rather than volumetric (bulb) pipettes. This also minimized the risk of contact with the solutions. Concurrent with the standards preparation, two alternate groups of 2 – 3 students each prepared the unknown for analysis by accurately weighing out ~0.25 g of bronze and then digesting these samples with aqua regia (10 mL 14 M HNO3 and 5 mL 12 M HCl). The addition of the acids to the bronze leads to an immediate change in the color of the solution (clear to green) and evolution of brown gas (NO2). This provides an opportunity to discuss the redox process occurring with oxidation of the metals to the relevant cations and reduction of the nitric acid. The digestion process takes several minutes.While the bronze is digesting, students are (re)acquainted with the flame test. Our approach has been to pre-prepare solutions of the ions of interest and place these in spray bottles that can be sprayed directly into the flame of a Meker burner. Many of the students are aware of the concept of atomic energy levels and some know that the colors arise from electronic transitions of the atoms in the flame. From this familiar background we build the idea of the colors being a form of analysis (emission spectroscopy). However, the exercise is designed to also highlight some limitations of this very simple form of detection. Firstly, through demonstration that not all metal atoms produce a visibly colored flame (e.g. tin). Secondly, through the inclusion of mixed solutions which demonstrate that the intensity of emission from one component may mask the presence of a second component. These results demonstrate to the students that visible emission spectroscopy is not suitable for analysis of tin in bronze and that it is necessary to apply a more advanced technique in the form of Atomic Absorption Spectroscopy. Following this there is a brief discussion on how absorption spectroscopy differs from emission spectroscopy. At this stage the digestion is usually complete so that this group of students can proceed to make up the bronze solution for analysis. The other team have usually prepared their calibration standards by this stage, and they are taken through the discussion of the application of the flame test.When both groups have completed preparing their relevant solutions then all the students are taken to the FAAS and introduced to the components of the instrument and its basic operation. The demonstrator sets up the instrument for analysis and then all students are given the opportunity to put their solutions through the FAAS beginning with the calibration standards, so that students can plot their calibration graph, and then analyzing the bronze sample(s). Generally there is some variability in the quality of the prepared standards using the adjustable pipettes but the inclusion of a replicate set of standards generally helps to smooth out any aberrations in the calibration plot. The analysis of replicate bronze samples also helps to provide a more reliable measure of the tin content of the sample.
2.4. Hazards
- Students were required to wear personal protective equipment (laboratory coat, lab glasses, and covered shoes) at all times in the laboratory and wore suitable gloves.Due to the time constraints, and unknown extent of laboratory experience of the students, the primary tin standard solution was pre-prepared and provided in labeled bottles. Students then only had to use the adjustable pipettes to transfer the required amounts of solution to 100 mL volumetric flasks.The concentrated acids used in this experiment are highly corrosive. To minimize the handling of acids these were delivered directly to the reaction flask using preset bottletop dispensers, which were placed in a fumehood.The flame tests were carried out using pre-prepared solutions in spray bottles. The Meker burner was placed in a spray booth in a fumehood so that there was minimal risk of inhaling the aerosol from the sprays during the flame test. Barium nitrate is a toxic compound and precautions should be taken to prevent ingestion of the solution.Standard precautions should be taken by staff preparing the solutions. The tin primary standard was prepared by digestion of a suitable quantity of tin metal using aqua regia.
3. Results
- Eight single metal ion and four binary metal ion solutions were investigated using the flame test (Table 1). For each of the binary metal solutions the colour from one of the metal ions present is generally sufficiently intense to mask the presence of the second cation.
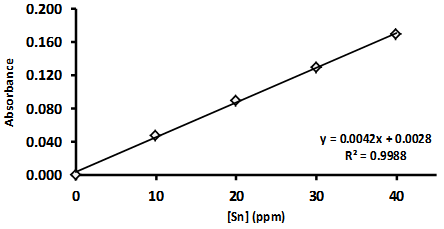 | Figure 1. A typical calibration plot for FAAS analysis of tin |
|
|
4. Discussion
- Through an increasing exposure to a range of science fiction and fictional crime shows, many students have developed an unrealistic expectation of the operation and capabilities of analytical instrumentation. [9, 10] These instruments are often considered as black boxes into which a randomly chosen sample can be placed for complete chemical or biological characterization. This experiment provides students with a greater understanding of the actual process, requirements and limitations involved in accurate analysis. We set the context for this by demonstrating to the students that simple qualitative techniques, such as the flame test, have their limitations including masking effects by some metals. Two copper solutions - a typical deep blue copper sulfate solution and a dark red copper MAC solution are also included to demonstrate that the flame colour is independent of the solution colour.Our experiment demonstrates that while an instrument can provide a numerical value for a characteristic, this value is often meaningless if it is not referenced against accurately known values for that characteristic. The students generally obtained quite good agreement with the expected result of 4 wt% tin and are able to conclude that the sample was a modern phosphor bronze, rather than a 17th century naval cannon. However, the similarity in the composition of these two bronzes demonstrates the need for care and precision in analysis. We also discuss with students how further investigation could focus on identifying if trace elements such as lead or phosphorous are present in the sample and how this might validate their initial conclusions.This experiment generates a discussion about the applicability of this analytical technique to an even greater range of situations including environmental monitoring, minerals assays, and some areas of biological and pharmaceutical analysis. Concepts such as oxidation and reduction, spectroscopy, data analysis, reporting to clients, etc. could be expanded upon quite easily.
5. Conclusions
- We have developed a simple and quick hands-on laboratory exercise, highlighting the connection between chemistry, spectroscopy and metallurgy. The experiment, based around tin analysis in bronze, has already been delivered to over 200 students and found to be suitable for use with high school students from the age of 15 up. The teamwork and investigative aspects of the exercise were valuable for stimulating discussion of not just this experiment but also the general concepts and uses of spectroscopic analysis.
ACKNOWLEDGEMENTS
- This experiment was developed as part of the ‘Extracting Talent for Metallurgy’ program for high school students, which is generously funded by Rio Tinto Australia. The authors thank Andrew Foreman, Tina Oteri, Saijel Jani, Aaron Brown and David Zeelenberg for their assistance in the development and initial testing of this experiment.
Appendix
- Bronze AnalysisThe smelting of copper and the manufacture of tools, weapons and coins from its alloy, bronze, began ~ 3300 BC. Ancient smelters, forges and mints generally relied on regional sources for ores and each had unique procedures for making bronze. Consequently there are predictable differences in the major and minor metals found in bronze tools, coins and weapons from different regions. The table below presents a brief summary of some of the variations found in the compositions of bronze objects.
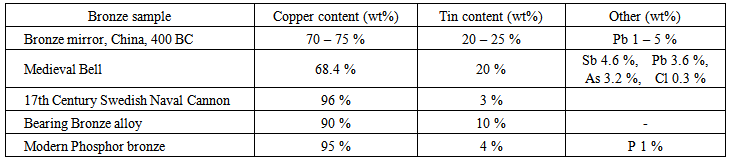 Chemical analysis can be used to determine the different types of metals within bronze objects and their relative ratios, to identify what regions and/or years they most likely came from or, alternatively, to identify if they are modern fakes. One valuable analytical chemistry technique is based on the behaviour of some materials when they are sprayed into a flame. Atoms placed in a flame absorb thermal energy, causing electrons within the atom to move from lower to higher energy states. When these electrons return to lower energy states, they emit energy in the form of electromagnetic waves (light). For some ions (such as those of alkali metals, alkaline earth metals, and some transition metals), the light emitted is in the visible spectrum and causes the flame to change color. Therefore flame tests can confirm the presence of specific metal cations in a sample. 1. Qualitative Flame analysisLight a Meker burner and adjust to produce a blue flame. Spray each of the metal ion solutions into the flame and record your observations in the table below.
Chemical analysis can be used to determine the different types of metals within bronze objects and their relative ratios, to identify what regions and/or years they most likely came from or, alternatively, to identify if they are modern fakes. One valuable analytical chemistry technique is based on the behaviour of some materials when they are sprayed into a flame. Atoms placed in a flame absorb thermal energy, causing electrons within the atom to move from lower to higher energy states. When these electrons return to lower energy states, they emit energy in the form of electromagnetic waves (light). For some ions (such as those of alkali metals, alkaline earth metals, and some transition metals), the light emitted is in the visible spectrum and causes the flame to change color. Therefore flame tests can confirm the presence of specific metal cations in a sample. 1. Qualitative Flame analysisLight a Meker burner and adjust to produce a blue flame. Spray each of the metal ion solutions into the flame and record your observations in the table below.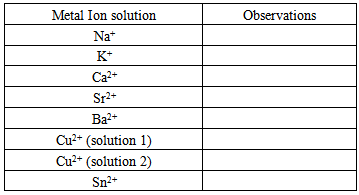 From your observations what metal ions are easy to detect using the flame test and what metals are difficult to detect? Now spray each of the mixed metal ion solutions into the flame and record your observations. Can you detect both metals using this test? What are some of the complications from this approach to analysis?
From your observations what metal ions are easy to detect using the flame test and what metals are difficult to detect? Now spray each of the mixed metal ion solutions into the flame and record your observations. Can you detect both metals using this test? What are some of the complications from this approach to analysis? Ancient bronze is an alloy of copper mainly with tin. The above qualitative test enables us to detect copper but not tin. A more advanced technique is therefore required to detect tin in unknown bronze samples.2. Flame Atomic Absorption Spectroscopy In the flame tests that you have just performed the metals in the solutions are identified by the color of light that they emit when excited in the flame. In this case the flame test is a very basic form of Emission Spectroscopy. An alternative method is to measure the amount of light absorbed by the sample using light with a wavelength that will be absorbed specifically by atoms of the metal of interest. This is the basis of Atomic Absorption Spectroscopy. In this experiment we will specifically be using Flame Atomic Absorption Spectrometer (FAAS) to easily and quickly measure the tin content in bronze samples.A schematic diagram of a FAAS is shown in Figure A1 below.
Ancient bronze is an alloy of copper mainly with tin. The above qualitative test enables us to detect copper but not tin. A more advanced technique is therefore required to detect tin in unknown bronze samples.2. Flame Atomic Absorption Spectroscopy In the flame tests that you have just performed the metals in the solutions are identified by the color of light that they emit when excited in the flame. In this case the flame test is a very basic form of Emission Spectroscopy. An alternative method is to measure the amount of light absorbed by the sample using light with a wavelength that will be absorbed specifically by atoms of the metal of interest. This is the basis of Atomic Absorption Spectroscopy. In this experiment we will specifically be using Flame Atomic Absorption Spectrometer (FAAS) to easily and quickly measure the tin content in bronze samples.A schematic diagram of a FAAS is shown in Figure A1 below.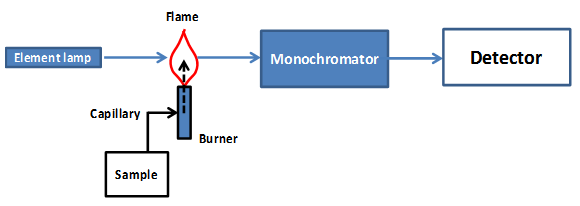 | Figure A1. Diagram of a Flame Atomic Absorption Spectrometer |
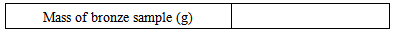 2. In the fume hood, add to the conical flask containing the bronze sample 10 mL of concentrated nitric acid and 5 mL of concentrated hydrochloric acid using the dispensers. Immediately, the solution will bubble violently and start to evolve a brown-orange gas. Contact with this toxic gas, NOx, must be avoided.3. The flask may become quite warm during the reaction so it should be set aside in a fumehood while the bronze dissolves. The solution should turn to a bright green color. 4. Once cool, carefully add approximately 50 mL of deionized water and swirl to dissolve any tin chloride that might have formed. Transfer this solution to a 500 mL volumetric flask. Make the solution up to the mark on the volumetric flask5. Take your sample to the FAAS for analysis.Groups 3 and 4: Preparation of Tin Standard SolutionsA 1000 ppm (1000 mg L-1) tin stock solution is provided. From this you need to prepare a series of calibration standards that cover the expected concentration range of the tin in the bronze solution.1. Prepare the 10 ppm calibration standard by pipetting 1 mL of the stock solution into a 100 mL volumetric flask and making it up to the mark with de-ionized water. Label the flask.2. Prepare the 20 ppm calibration standard by pipetting 2 mL of the stock solution into a 100 mL volumetric flask and making it up to the mark with de-ionized water. Label the flask.3. Prepare the 30 ppm calibration standard by pipetting 3 mL of the stock solution into a 100 mL volumetric flask and making it up to the mark with de-ionized water. Label the flask.4. Prepare the 40 ppm calibration standard by pipetting 4 mL of the stock solution into a 100 mL volumetric flask and making it up to the mark with de-ionized water. Label the flask.5. Take your calibration standards to the FAAS for analysis and record the absorbance for each solution.
2. In the fume hood, add to the conical flask containing the bronze sample 10 mL of concentrated nitric acid and 5 mL of concentrated hydrochloric acid using the dispensers. Immediately, the solution will bubble violently and start to evolve a brown-orange gas. Contact with this toxic gas, NOx, must be avoided.3. The flask may become quite warm during the reaction so it should be set aside in a fumehood while the bronze dissolves. The solution should turn to a bright green color. 4. Once cool, carefully add approximately 50 mL of deionized water and swirl to dissolve any tin chloride that might have formed. Transfer this solution to a 500 mL volumetric flask. Make the solution up to the mark on the volumetric flask5. Take your sample to the FAAS for analysis.Groups 3 and 4: Preparation of Tin Standard SolutionsA 1000 ppm (1000 mg L-1) tin stock solution is provided. From this you need to prepare a series of calibration standards that cover the expected concentration range of the tin in the bronze solution.1. Prepare the 10 ppm calibration standard by pipetting 1 mL of the stock solution into a 100 mL volumetric flask and making it up to the mark with de-ionized water. Label the flask.2. Prepare the 20 ppm calibration standard by pipetting 2 mL of the stock solution into a 100 mL volumetric flask and making it up to the mark with de-ionized water. Label the flask.3. Prepare the 30 ppm calibration standard by pipetting 3 mL of the stock solution into a 100 mL volumetric flask and making it up to the mark with de-ionized water. Label the flask.4. Prepare the 40 ppm calibration standard by pipetting 4 mL of the stock solution into a 100 mL volumetric flask and making it up to the mark with de-ionized water. Label the flask.5. Take your calibration standards to the FAAS for analysis and record the absorbance for each solution.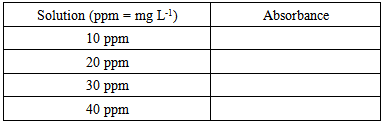 6. Plot a calibration graph from the above data.7. Using the above calibration graph and the absorbance values for the bronze solutions, determine the concentration of tin in the bronze solutions.8. Using the mass of you original sample and the measured concentration of tin in the bronze solutions determine the mass fraction of tin (wt%) in the original sample.Tin (wt%) = concentration of tin (mg L-1) x 0.5 (L) / (mass bronze (mg)) x 100Questions:How much variation was there between the different samples? What other metals could we determine in this way?How can we use this to determine the authenticity of a bronze artifact?
6. Plot a calibration graph from the above data.7. Using the above calibration graph and the absorbance values for the bronze solutions, determine the concentration of tin in the bronze solutions.8. Using the mass of you original sample and the measured concentration of tin in the bronze solutions determine the mass fraction of tin (wt%) in the original sample.Tin (wt%) = concentration of tin (mg L-1) x 0.5 (L) / (mass bronze (mg)) x 100Questions:How much variation was there between the different samples? What other metals could we determine in this way?How can we use this to determine the authenticity of a bronze artifact?
References
| [1] | Laird, D. W.; Henry, D. J. Is there Ni in my liquor? A Hands on Laboratory Exercise for Relating Chemistry to Extractive Metallurgy J. Chem. Ed. 2013, 90. 1671-1674. (http://pubs.acs.org/doi/abs/10.1021/ed400106m). |
| [2] | Soto, L.; Franey, J. P.; Graedel, T. E.; Kammlott, G. W. On the corrosion resistance of certain ancient Chinese bronze artifacts. Corrosion Sci. 1983, 23, 241-250.(http://www.sciencedirect.com/science/article/pii/0010938X83901051). |
| [3] | Ustohal, V.; Ptackova, M.; Prochazka, R. Composition of a medieval bell from a Brno church. Slevarenstvi, 1997, 45, 116-119. |
| [4] | Hallberg, R. O.; Östlund, P.; Wadsten, T. Inferences from a corrosion study of a bronze cannon, applied to high level nuclear waste disposal. Appl. Geochem. 1988, 3, 273-280. (http://www.sciencedirect.com/science/article/pii/0883292788901060). |
| [5] | Sanderow, H. I.; Pease, L. F., III. Capabilities of North American vs. European manufactured bronze bearings. Adv. Powder Metallurgy & Particulate Materials. 2004, 12, 49-58. |
| [6] | Donais, M. K.; Whissel, G.; Dumas, A.; Golden, K. Analyzing Lead Content in Ancient Bronze Coins by Flame Atomic Absorption Spectroscopy. An Archaeometry Laboratory with Nonscience Majors. J. Chem. Ed. 2009, 86, 343-346. (http://pubs.acs.org/doi/abs/10.1021/ed086p343). |
| [7] | Kharbade, B. V.; Agrawal, K. C. Determination of Phosphorus in Copper Artifacts by Graphite Furnace AAS. Atomic Spectros. 1997, 18, 130-131. |
| [8] | Beccaria, A. M.; Poggi, G. Analysis of Admiralty Brass Corrosion Products in Sea Water and Brackish Water. Analyst, 1986, 111, 959-963.(http://pubs.rsc.org/en/content/articlelanding/1986/an/an9861100959#!divAbstract). |
| [9] | Schweitzer, N.J.; Saks, M.J. The CSI Effect: Popular Fiction about Forensic Science Affects the Public’s Expectations about Real Forensic Science, Jurimetrics 2006-2007, 47, 357-364.(https://www.law.asu.edu/jurimetrics/JurimetricsJournal/ArticlesIssues/Abstracts.aspx#Vol47p357). |
| [10] | Bergslien, E. Teaching To Avoid the “CSI Effect”. J. Chem. Ed., 2006, 83, 690-691.(http://pubs.acs.org/doi/abs/10.1021/ed083p690). |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML