-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
2014; 2(4): 64-72
doi:10.5923/j.jlce.20140204.03
“Treasure the Exceptions”: An Investigation into why Heat-treated, Isolated Chloroplasts Fail to Respond to Uncoupling Reagents
Rob L. Dean
Department of Biology, The University of Western Ontario, London, Ontario N6A 5B7, Canada
Correspondence to: Rob L. Dean , Department of Biology, The University of Western Ontario, London, Ontario N6A 5B7, Canada.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Heat treated chloroplasts lose the ability to transfer electrons from the oxidation of water to a synthetic electron acceptor (DCPIP) but will still transfer electrons to the acceptor in the presence of an artificial electron donor (DPC). In many respects, heat treated chloroplasts respond predictably to a variety of experimental conditions but, unexpectedly, they do not respond to uncoupling reagents that usually greatly increase the rate of electron transport in untreated chloroplasts. An investigation of this unexpected result reveals that heat treatment not only prevents chloroplasts from oxidizing water but also uncouples the thylakoid membranes. Moreover, evidence is presented suggesting that heat treatment has one or more additional effects that reduce the rate at which electrons are transferred to the acceptor. This investigation of an unexpected result provides a useful lesson in how scientific progress is sometimes made by paying attention to experimental results that are not predicted.
Keywords: Isolated chloroplasts, Thylakoids, heat treatment, Uncoupling reagents, Dichlorophenol indophenol (DCPIP), gramicidin D, Diphenyl carbazide (DPC), Ammonium chloride, Electron transport rate
Cite this paper: Rob L. Dean , “Treasure the Exceptions”: An Investigation into why Heat-treated, Isolated Chloroplasts Fail to Respond to Uncoupling Reagents, Journal of Laboratory Chemical Education, Vol. 2 No. 4, 2014, pp. 64-72. doi: 10.5923/j.jlce.20140204.03.
Article Outline
1. Introduction
- I teach a section of a second year undergraduate laboratory course called Scientific Methods in Biology. We deal with experimental design, instrumentation, the evaluation of experimental data and scientific writing. Because the course is concerned with methods, the experimental systems we use are of secondary importance. However, the measurement of light-dependent rates of electron transport through the thylakoid membranes of isolated chloroplasts has proved to be a system that can be used to address a wide variety of experimental questions.Illuminated thylakoids oxidize water and the electrons liberated pass sequentially through photosystem II (PS II), plastoquinones, cytochrome b6/f, plastocyanin and photosystem I (PS I) to the final electron acceptor, NADP+ (see figure 1 in [1] for a schematic diagram). If 2, 6- dichlorophenol indophenol (DCPIP) is present in the reaction mixture, it intercepts electrons from the plastoquinones and becomes reduced. The rate of photoreduction of DCPIP by isolated chloroplasts is determined from the decline in absorbance at 600 nm over time.Previous work has shown that rates of electron transport from water to DCPIP by isolated chloroplasts increase with increasing irradiance [2], are sensitive to pH change and reduced in the presence of a herbicide [3] and increase when exposed to the uncoupling reagent, ammonium chloride [4]. A later investigative study [5] showed that mild heat treatment eliminates water oxidation so that no electrons are available to reduce DCPIP. It was further demonstrated that heat-treated chloroplasts will photoreduce DCPIP if an artificial electron donor, 1, 5-diphenyl carbazide (DPC), is included in the reaction mixture.Based on the assumption that electron transport through the thylakoid membranes of heat-treated chloroplasts follows the same pathway as that in untreated chloroplasts, a number of predictions about the behaviour of heat-treated chloroplasts under a variety of experimental regimes were made and tested. Almost all of the predictions were verified by experiment. For example, if electron transport from DPC to DCPIP in heat-treated chloroplasts follows the normal pathway from the oxidation of water to DCPIP found in untreated chloroplasts, the reaction should be sensitive to dichlorophenyl dimethyl urea (DCMU), a reagent that blocks the transfer of electrons from PS II to the plastoquinones. This hypotheses was experimentally supported [5]. There was, however, one puzzling result. Untreated chloroplasts photoreduce DCPIP at much higher rates in the presence of the uncoupling reagent, ammonium chloride (NH4Cl) than in its absence [4]. The ammonium ion is referred to as an uncoupling reagent because it eliminates the proton gradient that normally forms across the thylakoid membranes of chloroplasts as a consequence of electron transport through them. (It consequently “uncouples” electron transport from ATP synthesis or photophosphorylation). When the proton gradient is abolished, rates of electron transport through the membranes are maximized. However, the rate of electron flow from DPC to DCPIP in heat treated chloroplasts is not affected by ammonium ion; the process seems to be unresponsive to this uncoupling reagent. An investigation of this apparent anomaly is the basis of my paper.Laboratory education in experimental biology can be divided into two broad categories. The first is essentially algorithmic where students follow a set of instructions to reach a preordained result. This method can be useful for teaching techniques and reinforcing concepts covered in lectures. The second category is investigative. Here students use what is known to design experiments to test hypotheses that they construct with assistance from their instructors. The latter approach provides a better introduction to how research is conducted but, because time and resources are often limited, we often steer students towards experimental work in which the hypothesis is likely to be supported by the results. In this way, we completely ignore the importance of serendipity in the progress of science. A well-designed experiment in which the outcome is unexpected can open the door to a new understanding of the subject under investigation. Although students tend to regard such experimental outcomes as failures (“It didn’t work”), they should be encouraged to pay particular attention to anomalous results; to “treasure the exceptions” [6].The work reported here could therefore provide the background for an investigative study, but if resources and time are limited, the information can be added to the repertoire of prescriptive exercises for use in biologically-related laboratory classes.
2. Materials and Methods
2.1. Equipment
- Visible range spectrophotometers (e.g. Spectronic 20) and suitable cuvettes or tubes.Volumetric pipettes.Variable volume mechanical pipettes (10-100 µL range) with tips.Domestic blender.Clinical centrifuge with graduated tubes.Lamps with 100 watt frosted bulbs.Constant temperature circulating water bath.Cheesecloth, Parafilm.(A Li-Cor LI-189 quantum/radiometer/photometer with an attached LI-190 quantum sensor is very useful but not essential).
2.2. Stock Solutions
- Chloroplast Isolation Buffer: 50 mM tricine, 400 mM sorbitol, 10 mM NaCl, 2.5 mM MgCl2.6H2O,1.25 mM MnCl2, 0.3 mM Na2EDTA adjusted to pH 7.8 with saturated, aqueous NaOH.Chloroplast Reaction Buffer: (Prepared at 5x the concentration used). NaH2PO4 29.4 g/L, Na2HPO4,11.995 g/L and sorbitol 91.085 g/L. When diluted 5 fold in the reaction mixtures, this produces a 66 mM phosphate buffer containing 100 mM sorbitol (the osmoticum) at pH 6.3 with an ionic strength of 0.1 mM [7].80% (v/v) aqueous acetone.0.1 mM 2, 6-dichlorophenol indophenol (DCPIP) (BDH Chemicals).Dimethyl sulfoxide (DMSO) (Sigma).50 mM 1, 5-diphenyl carbazide (DPC) (Sigma) in DMSO.100 µM gramicidin D (Sigma) in DMSO (Molecular weight, not shown on the container, is ~ 2000).100 mM aqueous NH4Cl.
2.3. Chloroplast Isolation
- Weigh 25 g of spinach leaves from which the petioles and major veins have been removed. Cut the leaves into smaller fragments and place them in a blender cup that has been stored in the freezer. Add 100 ml of cold chloroplast isolation buffer and blend the leaves with three 5 second bursts at full speed.Filter the blended mixture (called a brei) through 4 layers of cheesecloth into a beaker on ice. Transfer six 10 ml aliquots into 15 mL capacity centrifuge tubes. Centrifuge for 5 minutes at 1300 x g. Discard the supernatant and add 2.0-2.5 mL of cold isolation buffer to each pellet. Resuspend the pellets with a camel hair paintbrush and pool them in a single tube. The resulting 12-15 mL of chloroplast preparation is enough for a minimum of 300 assays and can be stored on ice for hours with minimal loss of activity.
2.4. Estimation of the Chlorophyll Concentration of the Chloroplast Preparation
- Add 50 µL of chloroplast suspension to 5.0 mL of 80% acetone. Cover the tube with Parafilm and shake to dissolve the chlorophyll. Centrifuge at 1300 x g for 3 minutes. Read the absorbance of the supernatant at 652 nm (A652). The chlorophyll concentration, in mg chlorophyll/mL of chloroplast preparation, is calculated using equation 1 from reference [8].
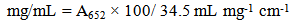 | (1) |
2.5. Heat (and lower Temperature) Treatment of the Chloroplast Preparation
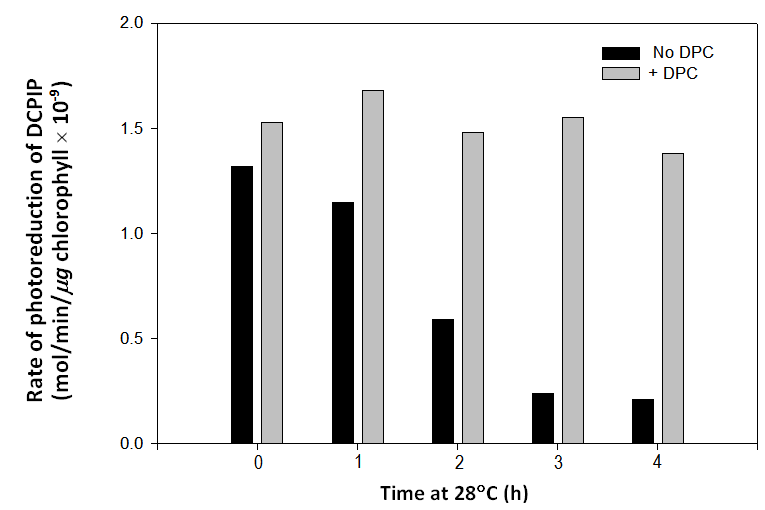
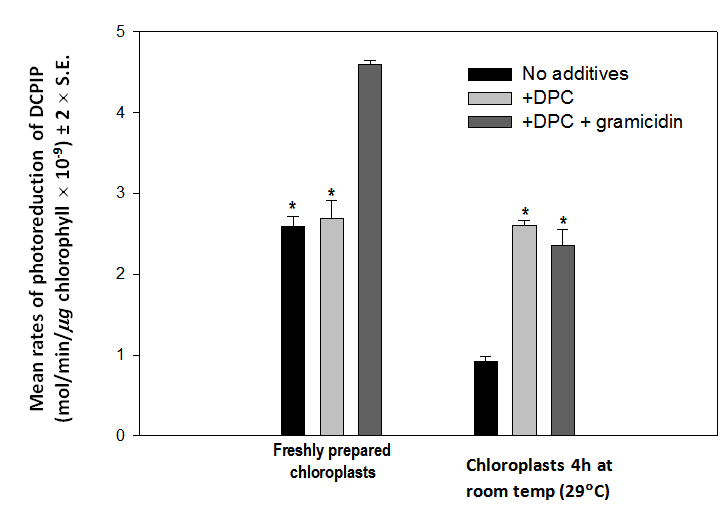
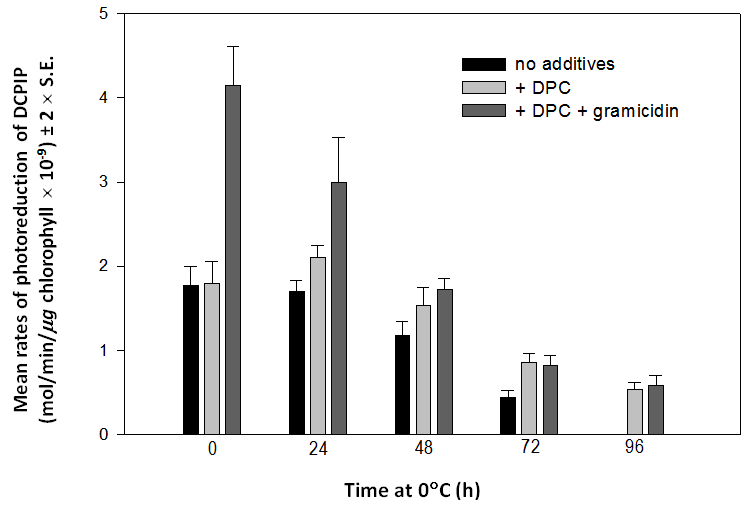
- One mL aliquots of fresh chloroplast suspension in 1.7 mL capacity disposable microcentrifuge tubes were floated in a plastic microfuge tube rack in a constant temperature, circulating water bath at 55C for 0 (untreated), 0.5, 1.0, 1.5 or 2.0 minutes. After heating, tubes were immediately cooled in ice-water.For lower temperature treatment, equally-sized aliquots of chloroplast suspension were maintained at room temperature (28 or 29C) for up to 4 hours or at 0C in ice-water in a refrigerator for up to 96 hours.
2.6. Reaction Mixtures
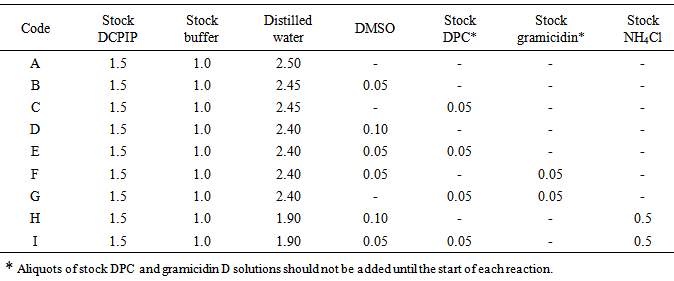
- All reaction mixtures, to which the chloroplast preparation was added, contained 30 µM DCPIP, 66 mM phosphate buffer (pH 6.3) and 100 mM sorbitol in a total volume of 5.0 mL. (Reaction mixture A, Table 1). Concentrations of the additional reagents in other reaction mixtures are summarized below and volumes of stock solutions required to make them are included in Table 1 (coded B to I).
|
2.7. Assay Protocol
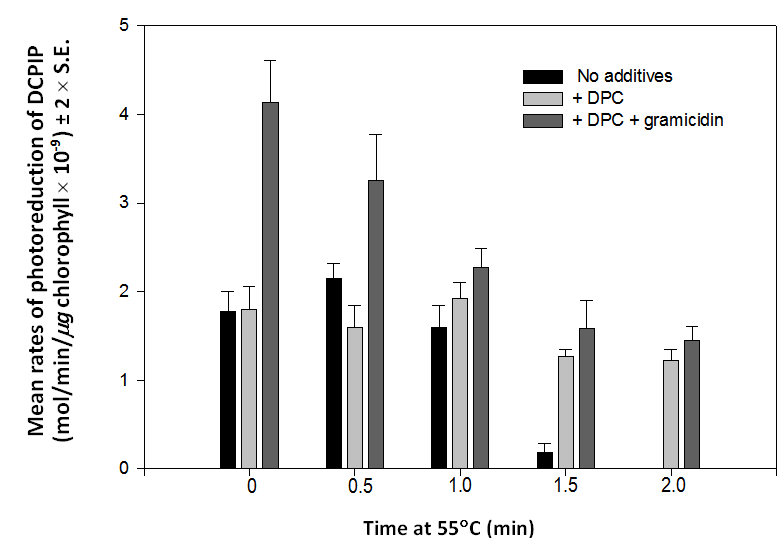
- Add a volume of chloroplast preparation containing the chosen quantity of chlorophyll to the reference blank, cap the tube with Parafilm and invert three times to mix. Use the blank to set the spectrophotometer to zero absorbance.The remainder of the work is carried out in a darkened room. For each reaction, in turn, add the chosen volume of chloroplast preparation, cap the tube and invert to mix. Take the initial A600 reading, place the tube in a beaker at the selected distance from the lamp and start timing when the lamp is turned on. Take A600 readings at 1, 2 and 3 minutes from the time the lamp was turned on. Return the tube to the beaker between readings. Dark controls are treated identically except that they are wrapped in aluminum foil between readings.Reaction mixtures containing NH4Cl can be made in bulk before running the reactions. In contrast, gramicidin D should not be added to the reaction mixture until the start of the reaction for reasons described elsewhere [9]. DPC should also be added at the start of the reaction because, at a slow rate, it chemically reduces DCPIP [5]. To start reactions containing these components, the chloroplast preparation is added first followed by the gramicidin D then the DPC. If, as is usually the case, only one mechanical pipette is available per student group, the pipette tips will need to be changed after each addition. Latex gloves should be worn when handling tips that have been in contact with DMSO because DMSO, and anything dissolved in it, is readily absorbed by human skin.In the work reported here, reactions were run at various distances from the light source. Results reported in Figures 1 and 2 were obtained at 10 cm, those in Figures 3, 4, 6 and 7 at 15 cm and those in Figure 5 at 20 cm from the lamp at photon fluence rates of 276, 196 and 139 µmoles of photons/m2/second respectively.
2.8. Rate Calculations
- As previously explained [4], the average rate during the first two minutes of the reactions produces satisfactory data. To calculate these rates:(a) Calculate the cumulative change in absorbance during the first two minutes (ΔA600/2 min) for each reaction by subtracting the A600 at 2 minutes from that at time zero.(b) Subtract the ΔA600/2 min for the dark control from the ΔA600/2 min for each of the reactions. This gives the corrected ΔA600/2 min for each reaction that reflects only the decline in absorbance due to the presence of illuminated chloroplasts.(c) The molar absorption coefficient (Є) is derived from the slope of a standard curve of A600 against DCPIP concentration (in moles/L) in the same buffer as is used for the reactions. The corrected ΔA600/2 min for each reaction is divided by the molar absorption coefficient to derive the change in concentration of DCPIP in the first two minutes of the reaction (Δc/2 min).(d) Divide the Δc/2 min by 2 to derive the Δc/min.(e) Correct for the total volume of the reaction mixture by multiplying the Δc/min by the total volume of the reaction mixture (i.e. 5.0 mL plus the volume of chloroplast preparation added) and dividing by 1000 ml/L. This gives the rate in terms of moles of DCPIP photoreduced/minute.(f) Divide the rate from step (f) by the number of µg of chlorophyll added to the reaction mixture to derive the final rate in moles of DCPIP photoreduced/min/µg of chlorophyll.
2.9. Statistical Analysis
- One-way analysis of variance (ANOVA) was conducted to compare treatment effects followed by Sheffe’s F-test to determine differences between means.
3. Results and Discussion
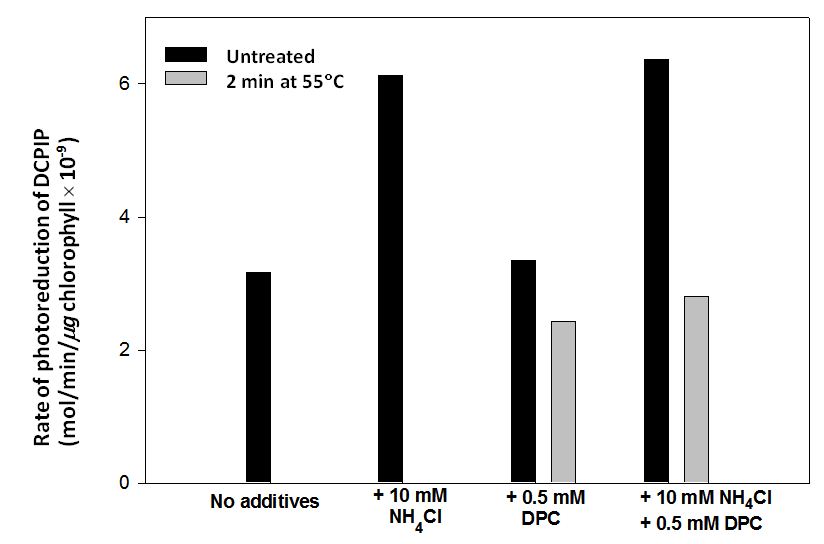
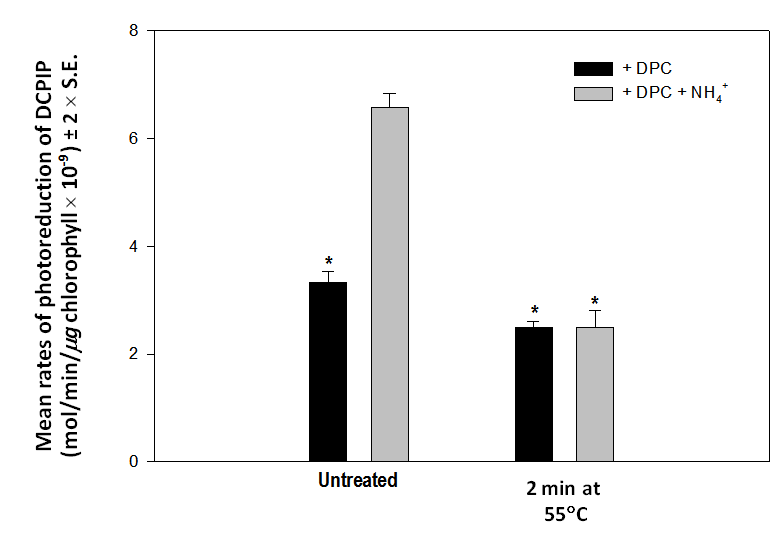
- The rate of photoreduction of DCPIP by untreated chloroplasts approximately doubles in the presence of 10 mM NH4Cl whether 0.5 mM DPC is included in the reaction mixture or not (Figure 1). This is the expected result in the presence of an uncoupling reagent [4]. Heat treated chloroplasts only photoreduce DCPIP when DPC is available to donate electrons to PS II (Figure 1). The rate of transfer of electrons from DPC to DCPIP by heat treated chloroplasts is little affected in the presence of 10 mM NH4Cl (Figure 1). This observation was confirmed in a statistical comparison of the response of untreated and heat treated chloroplasts in the presence of 0.5 mM DPC alone or DPC and 10 mM NH4Cl (Figure 2). Rates of photoreduction of DCPIP by heat treated chloroplasts in the presence and absence of NH4Cl are not significantly different (p > 0.05) (Figure 2). A detailed account of why NH4Cl enhances rates of photoreduction of DCPIP by untreated chloroplasts has been previously published [4]. To summarise briefly, ammonium ion in solution outside the thylakoid membrane dissociates into ammonia (NH3) and protons. The uncharged NH3 freely crosses the membrane and reassociates with protons within the thylakoid lumen to produce ammonium ion. Consequently, protons in the lumen that would have contributed to the proton gradient are sequestered in the ammonium ion (that is rapidly metabolized) and no gradient is generated. When no proton gradient is present, rates of electron transport through the electron carriers of the thylakoid membrane are maximal because no “back pressure” [9] of protons reduces the rate at which the plastoquinones can become oxidized.
4. Conclusions
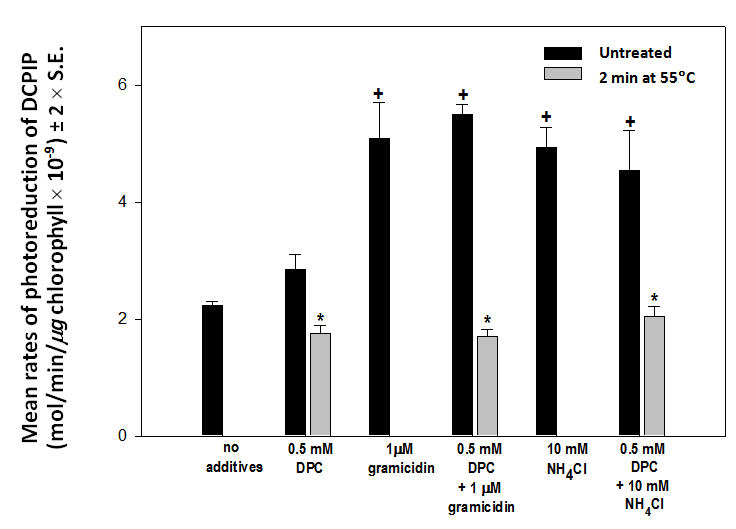
- A considerable quantity of experimental evidence previously led to the conclusion that, apart from their inability to oxidize water, heat treated chloroplasts are physiologically more or less normal [5]. However, a single unexpected observation, that heat treated chloroplasts do not respond to uncoupling reagents, led to this study that provides evidence that heat treatment may also completely uncouple the thylakoid membranes. This point makes it necessary to reevaluate all of the preceding data because it becomes apparent that heat treatment not only eliminates water oxidation and the formation of the proton gradient, but also has one or more other effects on the rate at which chloroplasts transfer electrons to DCPIP. The reason is as follows. Untreated chloroplasts, in which the electron transport is coupled to photophosphorylation, transfer electrons from water to DCPIP at a rate that, below light-saturation, depends on the irradiance level [2]. In the presence of an uncoupling reagent, untreated chloroplasts transfer electrons to DCPIP at a much higher rate [4]. At the same irradiance level we should expect enhanced rates of electron transfer from DPC to DCPIP in heat treated chloroplasts, because they are also uncoupled, but this is not the case. At a given irradiance level, rates of transfer of electrons from DPC to DCPIP by heat treated chloroplasts are usually about the same or lower than rates of electron transfer from water to DCPIP by untreated chloroplasts. Evidently some other aspect of the physiology of the chloroplasts has been affected by the heat treatment that places a limitation on the rates of electron transport through them. As is frequently the case in science, we appear to have resolved one problem and created another.In the normal course of a research project, investigations of unexpected data frequently require the use of totally different methods and equipment. Consequently, most teaching laboratories, in which materials and equipment are usually limited by budgetary considerations, are not set up to permit the further investigation of unexpected results. In this case, it was fortunate that the investigation of the possibility that heat treatment uncouples the thylakoid membrane only required a change in strategy; the methods and equipment were unchanged.Clearly, not all of the experimental work presented here can be carried out in a typical 3 hour laboratory class; the longer protocols would only be appropriate for students with unlimited access to the laboratory. However, variants of some of the protocols would provide interesting investigative studies within a typical laboratory session. For example, the response of chloroplasts to DPC alone and DPC plus gramicidin D after short periods at high temperature (Figure 4) could be easily accommodated. It would probably provide even more interesting results if chloroplasts were exposed to 55C for shorter times, e.g. 10, 20, 30, 40, 50 and 60 seconds. Similarly, exposing chloroplasts to 30 or 35C for 30 minute increments should be equally rewarding.The work described can also be the culmination of a series of related classes that simulate the approach that might be taken in a research laboratory. Exactly how this is done will depend on the available time. Our students spend two laboratory periods in preparation for working with isolated chloroplasts. The first class, an introduction to spectrophotometry, deals with the design of the instrument, how it works, the relationship between absorption and transmittance and the use of the instrument to generate absorption spectra and standard curves. In the second class, students are introduced to the principles of estimating reaction rates and gain experience by measuring the rate of reduction of DCPIP exposed to different initial concentrations of a chemical reducing agent. In these classes, students are assigned readings from our resource manual and a text that they use in a cell biology course. These readings cover light-dependent electron flow in thylakoid membranes, the consequent formation of a proton gradient across the membrane, how the proton gradient is abolished by uncoupling reagents, why the loss of the proton gradient increases the rate of electron transport to DCPIP, the effect of heat-treatment on water oxidation and the restoration of electron transport in heat-treated chloroplast exposed to DPC. This information is reviewed at the start of the third class and students then measure rates of photoreduction of DCPIP by isolated chloroplasts at different irradiance levels (i.e. distances from the light source) in the absence and presence of uncoupling reagents (ammonium ion and/or gramicidin D). In the fourth class, students investigate the hypothesis that heat-treated chloroplasts, using DPC as an electron donor, will respond to uncoupling reagents in the same way as untreated chloroplasts. Because of time constraints, this is as far as we can go in our course. However, it might be continued as follows. A class is probably needed to reflect on the data obtained thus far. In discussion with their instructors, students could generate possible explanations for the failure of heat-treated chloroplasts to respond to uncoupling reagents and design experiments such as, or similar to, those described in my paper.In whatever specific way this is undertaken, the phenomena I have described can provide students with an opportunity to further investigate an unexpected result without the need to refit the laboratory and to learn from first hand experience that negative or unexpected results can be important in advancing scientific knowledge.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML