-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
2014; 2(4): 58-63
doi:10.5923/j.jlce.20140204.02
Theoretical Explanation of Vulcanisation of Latex of Landolphia Owariensis (P. Beauv) Using Sodium Sulphite (Na2SO3) Solution Under Low Temperature
Eugene U. Okorie
Department of Science Education, University of Nigeria, Nsukka, Enugu State, Nigeria
Correspondence to: Eugene U. Okorie, Department of Science Education, University of Nigeria, Nsukka, Enugu State, Nigeria.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
This paper offers a theoretical explanation of vulcanisation of latex of Landolphia Owariensis (P.Beauv) using sodium sulphite (Na2SO3) solution under low temperature. The author proposes reaction routes in vulcanising the latex and suggests a structure for vulcanised rubber. The study set out to answer the question: what happens in chemical reactions involving natural rubber of which the latex of Landolphia Owariensis (P. Beauv) is composed, and what is the reaction mechanism during its vulcanisation? Latex of Landolphia Owariensis (P. Beauv) is one of the local plant materials identified in Nigeria for teaching some Organic Chemistry concepts in secondary schools. The latex has been used in a laboratory-based experiment to teach secondary schools students the concept of vulcanisation of rubber, but not with the conventional method of vulcanisation.
Keywords: Theoretical Explanation, Vulcanisation, Latex of Landolphia Owariensis (P. Beauv)
Cite this paper: Eugene U. Okorie, Theoretical Explanation of Vulcanisation of Latex of Landolphia Owariensis (P. Beauv) Using Sodium Sulphite (Na2SO3) Solution Under Low Temperature, Journal of Laboratory Chemical Education, Vol. 2 No. 4, 2014, pp. 58-63. doi: 10.5923/j.jlce.20140204.02.
Article Outline
1. Introduction
- In the last couple of decades, education in Chemistry in Africa and particularly Nigeria has placed emplaces on the use of local materials and resources. This is a way of ensuring cost-effectiveness of chemistry education in the face of rapid socio-cultural and economic changes, which most nations in the African continent are undergoing. For this reason, secondary school chemistry curriculum planners insist that for the curriculum objectives to be achieved, chemistry educators should situate the implementation of the curriculum in the immediate environment of the students; and incorporate during lesson preparation suitable local materials, which will not only awaken in the students the spirit of enquiry, but also help them to appreciate the need to acquire relevant chemical knowledge and skills [1].One of the local materials identified in Nigeria for teaching some Organic Chemistry concepts is latex obtained from a plant called ‘uke’ or ‘ụtụ’ (Igbo). The plant is found in different locations in the South-eastern states of Nigeria, especially in Alayi (Abia State) and Owerri (Imo State), where it is distributed in large quantities. The plant was first identified and described in Owerri in 1804 by Landolphia P. Beauv [2]. Since then the plant has assumed the botanical name, Landolphia Owariensis (P. Beauv), and it belongs to the family of Apocynaceae. A survey of the geographical spread of the plant showed that the spread extents through Cameroun and Equatorial Africa to Egypt, Sudan, Uganda and southern Tanzania. In the West African sub-region, Landolphia Owariensis (P. Beauv) is found in various locations in Guinea, Sierra Leone, Liberia, Ivory Coast, Ghana, and Togo [2], [3]. The local names of the plant in various parts of Africa are documented [4].The plant produces copious white latex [2], [3], [5]. The latex of Landolphia Owariensis (P. Beauv) looks similar to that of common West African rubber tree.
2. Latex of Landolphia Owariensis as a Local Chemical Material
- Okorie [6] worked on synthesis of polymer using latex of Landolphia Owariensis (P. Beauv) as a local material and asserted that the material could be used as a chemical compound for teaching some chemical concepts: un-saturation, vulcanisation and polymerisation. To justify this assertion, school laboratory-based experiments were devised [7], [8] to illustrate how teachers could use the local chemical materials in teaching the following concepts:1. Test for un-saturation;2. Identification of the type of bond present in unsaturated compound.
2.1. Chemistry of Latex
- Latex is a suspension of microscopic particles of natural rubber in an aqueous solution. Natural rubber essentially is an unsaturated compound composed mainly of the polymer, 2-methybta-1, 3-diene and has CH2=C (CH3)-CH=CH2 as its structural formula [9], [10], [11], [12], [13]. It adds bromine and hydrogen bromide and undergoes catalytic hydrogenation. By sulphur treatment (vulcanisation), an elastic material is produced.
3. Chemistry of Latex of Landolphia Owariensis (P. Beauv)
- Theoretically, the chemistry of the rubber latex is that of an ethylenic (alkene) material. The chemistry of natural rubber latex has been studied in greater details. It is noted that as an unsaturated compound, the rubber is shown on analysis to contain one double bond per C5H8 group and conclusion form studies shows the rubber to be built up by addition of 2-methylbuta-1, 3–diene units and has the structure shown below:
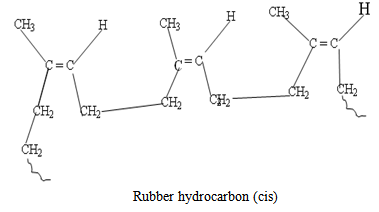 The rubber molecule undergoes characteristic addition reactions of unsaturated compounds, but this is often followed by substitution and/or cyclisation [9]. Cyclisation is a reaction, which results in a long polymer chain recurring in cycles or rings. The rubber can be vulcanized.
The rubber molecule undergoes characteristic addition reactions of unsaturated compounds, but this is often followed by substitution and/or cyclisation [9]. Cyclisation is a reaction, which results in a long polymer chain recurring in cycles or rings. The rubber can be vulcanized.3.1. Chemical Reactions of Latex of Landolphia Owariensis (P. Beauv)
- Latex of Landolphia Owariensis (P. Beauv) is assumed to be composed mainly of 2-methylbuta-1, 3-diene. The compound is capable of undergoing cyclisation, saturation and vulcanisation in addition to other reactions under appropriate conditions. The problem here is to determine the mechanism and the actual chemical reactions of the latex during its vulcanisation under low temperature and in liquid phase. The reactions of latex of Landlophia owariensis (P. Beauv) are those of alkenes. The alkenes mainly undergo addition reactions often leading to the saturation of the compounds. However, the chemistry of latex of Landlophia owariensis (P. Beauv) presents yet another dimension, which is unique to the rubber compound. As a typical alkene material, the latex undergoes very many varieties of reactions involving the carbon-carbon pi
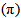 bond, and has reactive sites in the macromolecular chain, namely the carbon atom alpha
bond, and has reactive sites in the macromolecular chain, namely the carbon atom alpha 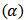 to the double bonds. The hydrogen atoms at these sites are readily removed giving rise to highly reactive radicals. Most chemical reactions involving the double bonds of alkenes amount to addition of reagents, X-Y, across the pi bond resulting, in a saturated molecule as shown below [9]:
to the double bonds. The hydrogen atoms at these sites are readily removed giving rise to highly reactive radicals. Most chemical reactions involving the double bonds of alkenes amount to addition of reagents, X-Y, across the pi bond resulting, in a saturated molecule as shown below [9]: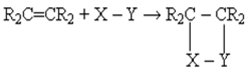 | (1) |
3.2. Reaction Conditions Which Favour Electrophilic and Free Radical Additions
- Addition of a reagent, X – Y, to the alkene that involves a free radical stepwise process is favoured by the presence of a radical initiator or if the reaction mixture is exposed to light, the addition reaction can be achieved. Reaction conditions, which favour electrophilic additions include low temperature, use of polar solvents such as water and alcohols, presence of ionic salts like sodium or potassium bromide in the bromination reaction, and the absence of light and of peroxide. In contrast, the free radical addition is favoured by performing the reaction in the gas phase or in non-polar solvents in the presence of very strong light or peroxide [14].
3.3. Rotation of Intermediate Products of Addition Reactions
- Intermediates result from the addition of a nucleophile and of a free radical, respectively, to an alkene. These intermediates are free to undergo rotation [14] about the single bond between the carbons that were originally joined by a double bond.
3.4. Summary of Review
- The review so far has been on explication of the nature of the type of reactions, which the alkene structure of the latex of Landolphia owariensis (P. Beauv) undergoes. The reaction conditions that are needed in some of these reactions were discussed. In summary, alkenes undergo mainly addition reactions resulting in saturation of the compound. The rubber molecule undergoes this ethylenic reaction (in the form of electrophilic, nucleophillic and free radical additions), but this reaction is often complicated by side reactions involving substitutions and / or cyclisation. The intermediates, which are generated by addition of nucleophile and a free radical respectively to an alkene, are free to undergo rotation about the single bond between the carbon which were originally joined by a double bond. It is precisely through this reaction mechanism that the rubber molecules undergo cyclisation. The essence of highlighting the above details is to indicate not only what happens in chemical reactions involving rubber latex of which the latex of Landolphia Owariensis (P. Beauv) is composed, but also how they happen, that is, the suggested reaction mechanism. The aim is to examine how this knowledge of the reactions has been employed in the vulcanisation of the rubber in the conventional way. This is with a view to seeing which other method could be devised in effecting the same chemical reaction, and perhaps under different experimental conditions designed to teach the concept to students in secondary school laboratories, especially with their attendant inadequacies. Many of the secondary school chemistry laboratories are ill equipped. It is on these well-established facts that the school laboratory-based experiments using the latex of Landolphia Owariensis (P. Beauv) were designed and carried out.
3.5. Vulcanisation of Rubber
- The conventional method of vulcanising natural rubber involves the formation of intermolecular bonds cross-linking using sulphur, which is the most widely used vulcanising agent. Sulphur is employed in conjunction with an accelerator at about 150-200℃.Charles Goodyear who was the first person to incorporate sulphur into masticated rubber and found that, after heating and cooling, the product became flexible and elastic discovered vulcanisation in 1840. Vulcanisation is brought about mainly by addition of a chemical, such as sulphur, to the masticated rubber and, when this mixture becomes homogenous, it is then placed into a mould and heated, to above 110℃ in the case of sulphur, the rubber molecules then become cross-linked. The hardness of the final product depends on the level of cross-linkage.The important thing to note here is that in the conventional process of vulcanisation of rubber, solid sulphur is used and a temperature range of 150-200℃ is required to bring about the cross-linkage. Again, rubber has to be in the ‘dry’ form such that it could be masticated. Therefore, the conventional method does not employ the rubber in its latex form.
3.6. Aim of this Study
- The aim of the present study is to offer a theoretical explanation of the reaction resulting in vulcanisation of the latex of Landolphia Owariensis (P. Beauv) using sodium sulphite solution under low temperature.
3.7. Research Questions
- The questions this study set out to answer are:1. How can rubber be vulcanised in its latex form, with out first drying it to the solid form?2. Is there no other way of vulcanising the rubber without using solid sulphur and high temperature?3. How can vulcanisation be carried out under lower temperature and in liquid phase to achieve expected result?4. Finally, what are the reaction routes involved in vul-canisation of the latex of Landolphia Owariensis (P. Beauv) using sodium sulphite solution under low temperature?
3.8. Significance of the Study
- Answers to these questions have a lot of implication for chemistry teaching. For instance, the conventional method of vulcanisation is mainly an industrial process, which may not be easily carried out in ordinary secondary school laboratory. This means that students cannot easily learn and appreciate the concept without first being exposed to industrial experiences. Therefore, there is need to search for a new method of achieving this same chemical reaction in the secondary school laboratory, in order to teach this chemical concept without having recourse to industries, which are not readily accessible.In this present study, attempt is made to ascertain the effectiveness of the school laboratory-based experiments designed by the researcher to utilise the latex of Landolphia Owariensis (P. Beauv), as an alternative laboratory chemical and local material in teaching the chemical concept of vulcanisation to secondary school students. The detail of the method employed is described below:First, the latex of Landolphia Owariensis (P. Beauv) was tested for un-saturation using the common elementary chemistry method. The test proved positive. A further test for identification of type of bond present in the compound confirmed the presence of double bond. Presence of triple bond in the compound would have resulted in white precipitate of silver acetylide, Ag-C≡C-Ag, (a confirmatory test for the presence of triple bond), but this was not observed.The structure of 2-methybuta-1, 3-diene was therefore assumed in the present study as the confirmed structure of the latex of Landolphia Owariensis (P. Beauv). It is on this assumption that the reaction routes, discussions and prediction in this study are based.
4. Vulcanisation of Latex of Landolphia Owariensis (P. Beauv)
4.1. Choice of Reagent
- Since all natural rubbers can be vulcanised, it is assumed in this experiment that the latex of Landolphia Owariensis (P. Beauv) being composed of the same polymer structures with those of rubber could as well be vulcanised. Vulcanisation is essentially the incorporation of sulphur into the rubber molecule. One possible way of doing this is to heat solid sulphur together with the latex; the other method is to use sulphur-containing salt. The conventional method requires much heat to bring sulphur to the vapour phase to facilitate its incorporation into the latex. Sulphur requires about 113℃ to melt and this temperature is high enough to derubberise the latex. Derubberise in the context of this study is construed to mean causing a fundamental change in the rubber molecule of which the latex is composed of, resulting in the destruction of the physiological activities of the molecule.This researcher wanted to work with a temperature of between 90℃ and 100℃, and in liquid phase (of polar solvent), because a situation in which the reaction could be carried out in solution and under minimum temperature is most desirable. It has two advantages: the reaction is faster and no damage is done to the old and new single bonds once formed and it eliminates free radical initiators which might result in the formation of different product other than the one desired.A better method therefore for the vulcanisation of the latex should be the use of sulphur-containing salt, which can be made into solution with water. Sulphur-containing reagents also owe their usefulness in part to the capacity of sulphur to utilise 3d orbitals for bonding and to occur in valence state higher than 2.The common sulphur-containing salts, which can be made into solutions and worked with in the liquid phase, include:1. Sodium thiosulphate (Na2S2O3)2. Sodium sulphite (Na2SO3)This researcher worked with the two salts in separate reactions and found the two materials excellent for the desired reaction. The two materials show similar reactions. For instance, the reaction between sodium thiosulphate and hydrochloric acid takes a noticeable time. Sulphur is produced during the reaction making the reaction cloudy. The equation of the reaction can be written as follows:
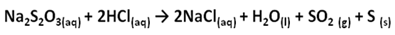 | (1) |
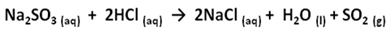 | (2) |
4.2. Experimental Procedure in vulcanisation of Latex of Landolphia Owariensis (P. Beauv)
- A portion of the latex was mixed with 2M dilute hydrochloric acid, sodium thiosulphate solution in the ratio 1:1:3 W/W and heated in the beaker to boil under normal laboratory condition. The latex coagulated and became tough and elastic. The vulcanised rubber was removed and washed with cold water.
4.3. Chemical Reaction
- The chemical reaction involved in the above experiment is explained here in the light of the theories already advanced by various researchers and the various laboratory observations made in this study. First, the chemical reaction was thought to be essentially the incorporation of sulphur into the rubber hydrocarbon molecules, which later resulted in cross-linkage and cyclisation.
4.4. Reaction Route
- The process in this reaction was assumed to have taken various steps as discussed below: Step I. Addition reaction leading to the hydrochlorination of the C=C bond and saturation of the alkene structure. (equation (3).
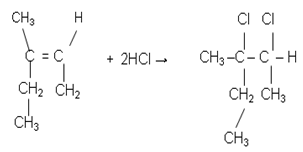 | (3) |
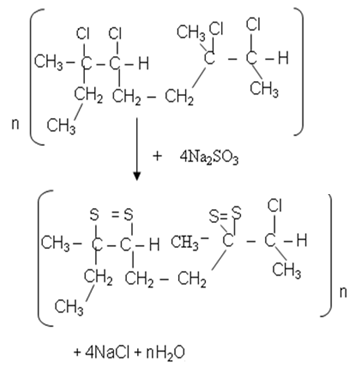 | (4) |
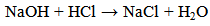 | (5) |
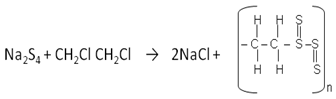 | (6) |
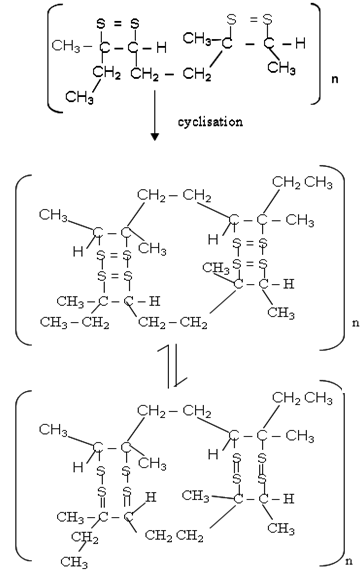 | (7) |
5. Suggestion for Further Studies
- The interest of this study is to offer a theoretical explanation of the vulcanisation reaction involving the latex of landolphia Owariensis (P. Beauv) and sodium sulphite under low temperature. Therefore, the determination of the actual and final structure of the vulcanised rubber is beyond the scope of this study. The determination of the true structure of this final structure of vulcanised rubber presents a research topic for further investigation.
ACKNOWLEDGEMENTS
- I am grateful to Professor G. O. M. Onwu who supervised my masters degree programme at the university of Ibadan, which gave rise to this paper.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML