-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
2014; 2(1): 4-9
doi:10.5923/j.jlce.20140201.02
Developing an Environmental Analysis of Soils and Water by Spectrochemical Techniques for Undergraduate Students of Chemistry
Jesús Anzano, LeyreAbia, Minerva Aragonés, Elena Ballano, Beatriz Guzmán, Mª Luisa Lomero, Catalina Pena, Elisa Pérez
Laser laboratory, Department of Analytical Chemistry, Faculty of Sciences, University of Zaragoza, Pedro Cerbuna #12, 50009, Zaragoza, Spain
Correspondence to: Jesús Anzano, Laser laboratory, Department of Analytical Chemistry, Faculty of Sciences, University of Zaragoza, Pedro Cerbuna #12, 50009, Zaragoza, Spain.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
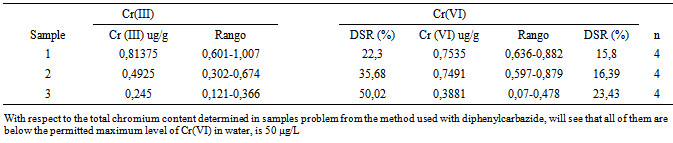
Quantification of heavy metals (Cr, Cu, Zn, K, Ca, Fe and Mn) in water and soils samples was performed through atomic and molecular spectrometry. The validity of three leaching agents (ethylenediaminetetraacetic acid, acetic acid and deionized water) was compared in soil samples to potassium, calcium, iron and manganese by means of atomic spectrometry. Acetic acid was the best leaching agent for all metals except for iron, and EDTA was the second best.Two experiments were developed for water samples. First, precipitation was used to separate toxic metals and, secondly, the speciation of Cr (III) and Cr (VI) was carried out using diphenylcarbazide and ethylenediaminetetraacetic acid to set up complexes selectively. It was proved that the removal of Cr, Cu and Zn by precipitation of the hydroxide was effective obtaining low ranks.
Keywords: Atomic and Molecular Spectroscopy, Determination, Potassium, Calcium, Iron, Manganese, Chrome, Cupper, Zinc, Soil, Water
Cite this paper: Jesús Anzano, LeyreAbia, Minerva Aragonés, Elena Ballano, Beatriz Guzmán, Mª Luisa Lomero, Catalina Pena, Elisa Pérez, Developing an Environmental Analysis of Soils and Water by Spectrochemical Techniques for Undergraduate Students of Chemistry, Journal of Laboratory Chemical Education, Vol. 2 No. 1, 2014, pp. 4-9. doi: 10.5923/j.jlce.20140201.02.
Article Outline
1. Introduction
- Nowadays, the presence of heavy metals in the environment is a very interesting issue, due to their persistence in soils and waters[1]. Considerable amounts of polluting metals are accumulated yearly. Soil and water are among the valuable common properties societies try to preserve the most, but they can be polluted by household, industrial or farming wastes. Heavy metals are among the polluting substances, usually coming from industrial processes such as paint production or chroming[2]. From a chemical point of view, heavy metals contain elements known as transition and post-transition. Those elements are significantly heavier than light metals like K or Na. Some of them are animal and plant nutrients but they are toxic in high concentrations. For example, trivalent chromium is an essential oligoelement and its deficiency causes diabetes and other adverse effects in the body. However, hexavalent chromium is extremely toxic, as it causes allergy, breath and liver deficiency, mutations and cancer[3]. Because of these harmful effects in the long and short-term, maximum admissible concentrations of the seions in drinking water, publicor industrial discharges must be regulated by the applicable laws. Toxic concentration limits are different to each metal and depend on the kind of ion and its physiological and environmental effects.European law (Directive 98/83/CE, 3 of November of 1998, relating to drinking water quality published in DOCE L Nº 330, 5 of December of 1998) fix chromium (0.05 mg/L) and cupper limits (2 mg/L) in drinking water. Zinc does not have risk level in water but World Health Organization lay down 5 mg/L as a legal limit. On the other hand, law 22/2011 (28 of July, of wastes and contaminated soils) and law 1/2005 (4 of February, of prevention and correction to soils contamination) regulate contaminated agents in soils[4, 5].Analytic techniques for metal analysis in soils and water samples are varied; their election depends on the metal concentration grade and its nature. Usual techniques are: Flame Atomic Absorption Spectrometry (AAS-Flame), Graphite Furnace Atomic Absorption Spectrometry (GFAAS), Inductively Coupled Plasma - Atomic Emission Spectrometer (ICP-AES), Inductively Coupled Plasma-Mass Spectrometry (ICP-MS). Multielement analysis and automatic techniques result particularly useful because they generate big data series and they need minimum effort and time[6].This article contains two different parts: metal determination in soils and in water. In our case, we search calcium, iron, manganese and potassium concentrations in soil samples. We make a leaching with different extract agents (ethylenediaminetetraacetic acid, acetic acid and deionized water) and we quantify each metal by Atomic Emission Spectrometry (AES) and Atomic Absorption Spectrometry (AAS).Apart from this method, others have been published, which are effective to heavy metals elimination. Two of them are Cr (III) extraction with coal[7]; and the use of modified polymers which allow the division of selectively toxic ions such as Pb (II), Cd (II) or Ni (II)[8]. Other example is biosorption metal elimination using biomass that is a field on the increase[9, 10].Otherwise, we proceeded to the separation, removal and quantification of Cr (VI), Zn (II) and Cu (II) in wastewater by precipitation with pH control and AAS-flame, and then we proceeded to the specific determination of chromium in dissolution problem.Spectrophotometric techniques are commonly used as a UV-visible absorption, chemiluminescence and fluorimetry because they allow making a selective determination of metallic species by means of the use of reactive that creates absorbent chemical species selectively.The most common method to determine Cr (VI) in aqueous solution is based on the selective reaction of diphenylcarbazide (DPC) to Cr (VI) to pH ~ 1[11]. This reaction has also been used for the extraction of chromium from their samples by techniques such as ion chromatography or solid phase extraction. This reaction forms a colored complex which absorbs in the visible (λ = 542 nm), the absorbance of which can be related to the concentration of Cr (VI) by the Beer-Lambert law. For performance an analysis of total chromium content of a sample by this method had to be made a pre-oxidation of the sample, the actual concentration of Cr (III) was obtained by difference between the concentration of total chromium and Cr (VI). Thus, our goal was the determination of Cr (III) and Cr (VI) in a test solution UV-visible spectroscopy using diphenylcarbazid.This article is developed at the Faculty of Sciences, University of Zaragoza[12]. The target students belong to the internship done in the fourth course of the Bachelor’s degree in Chemistry.
2. Experimental
2.1. Apparatus
- A Perkin-Elmer Model 2380 atomic absorption/emission spectrometer, fitted with Perkin-Elmer (calcium, manganese, iron, chromium, copper, zinc) hollow-cathode lamps was used for measure the contents of metals in soils and waste waters. Calcium, manganese, iron, chromium, copper and zinc were determined by Flame-AAS, while potassium was determined by Flame-AES (no lamp required).The instrumental parameters used in measuring the absorption/ emission of the studied elements are given in the table 1.
|
2.2. Reagents
- The reagents used for the analysis of soils were: Acetic Acid (0.43M) [Panreac, http://www.panreac.es/], EDTA (0.05M) [Panreac], Nitric Acid[Panreac].Calcium standard solution, 1000μg ml-l. Prepared by dissolving 2.52 g of calcium carbonate in 50 mI of 1MHCI and diluting to 11 with water.Manganese solution, 1000μg ml-l. Prepared by dissolving 3.25 g of manganese nitrate in 50 mI of 1MHCI and diluting to 11 with water.Iron solution, 1000μg ml-l. Prepared by dissolving 4.33 g of iron nitrate in 50 mI of 1MHCI and diluting to 11 with water.Potassium solution, 1000 μg ml-l. Prepared by dissolving 2.6 g of potassium nitrate in 50 mI of 1MHCI and diluting to 11 with water.All solutions were prepared with analytical-reagent grade chemicals and re-distilled water. They were kept in polyethylene containers. This solution is stable for at least 2 months. Solutions of lower concentrations of these three metals were prepared each dar by diluting the standard solutions.The reagents used for the analysis of water were: Sulfuric Acid [Panreac], Calcium Chloride [Panreac], Sodium Hydroxide [Merck, http://www.merck.es], Ascorbicacid [Panreac], Diphenylcarbazide [Scharlau], Acrylamide, Potassium manganate (VII)[Scharlau]The solutions were prepared with analytical-reagent grade chemicals and distilled water.Chromium solution 1000 mg/L, dissolve 0.283 g of potassium dichromate in 50 mL.[Panreac].Copper solution 1000 mg/L, dissolve 0.3862 g of copper (II) nitrate in 50 mL. [Panreac].Zinc solution 1000 mg/L, dissolve 0.2830 g of zinc (II) nitrate in 50 mL. [Panreac].
2.3. Samples
- Soil Sample: Commercial fertilizer (Auchan, http://www.simply.es)Humidity (105ºC) = 50±10%; pH = 5.5-6.5; Mineral fertilizers: (N)=220mg/l ± 10%; (P2O5)= 225mg/l ± 10%; (K) => K2O= 300mg/l ± 10%.Wastewater Samples:Provided by the Analytical Chemistry Department of University of Zaragoza.
2.4. Procedure
- Humidity of soil sample determination.-Approximately 1g of soil sample is weighted and then it was dried in a stove (~105ºC) until no change of weight is observed. The percentage of humidity will be useful later to refer the results of metal content to the dry sample.Extraction with EDTA.-A solution (200 mL) of EDTA 0,05M is prepared from the salt, and is adjusted to pH 7 by the addition of diluted ammonia and 5g of soil sample are weighted inside a plastic container and 50 mL of EDTA 0,05M are added. The contents of the container are stirred vigorously for 1h at room temperature. The extract is decanted and filtered, being collected into a 50 mL flask.Extraction with acetic acid.-A solution (500 mL) of HAc 0,05M is prepared from commercial HAc and 5g of soil sample are weighted inside a plastic container and 200 mL of HAc 0,05M are added.The contents of the container are stirred vigorously for 24h at room temperature.The extract is decanted and filtered, being collected into a 50 mL flask with 2 mL of HNO3 (conc.).Extraction with water.-40g of soil sample are weighted inside a plastic container and 100 mL of distilled water are added. The contents of the container are stirred vigorously for 2h at room temperature. The extract is decanted and filtered, being collected into a 50 mL flask with 2 mL of HNO3 (conc.).Determination of K, Ca, Fe, Mn using calibration graph.- Using the stock solutions for each metal, intermediate dilutions are prepared. These intermediate dilutions are diluted further to prepare series of calibration standards in the range of 1 to 10 mg/L of the metal. The range and number of “points” for the calibration may vary between metals. Using the appropriate wavelength in the spectrometer, the absorption of the calibration standards is measured to provide the corresponding calibration curves. Then, the absorption of the extracted samples is measured and interpolated.Determination of Cr, Cu, Zn by Atomic Absorption SpectometrySample treatment-Transfer 20 mL of the sample into 5 glasswares and acidify them to pH 2.98 (measuring with a pHmeter). Add a few milligrams of ascorbic acid and 3 mL of CaCl2 solution which will act as a co-precipitate. Adjust the solutions again using the pH meter to different pH with a NaOH solution: 6.3, 8.4, 10.78 and 12. One of them will remain at pH 2.98. Finally add a few milligrams of acrylamide, a clarifying agent. Let the samples rest for 30 minutes and then filter them to separate the precipitate. Wash the solid with water. Transfer the solutions into 100 mL flasks and make them up to volume with distilled water.Preparation of calibration standards and blank. Using the stock solutions for each metal, intermediate dilutions are prepared. These intermediate dilutions are diluted further to prepare series of calibration standards in the range of 1 to 10 mg/L of the metal. The range and number of “points” for the calibration may vary between metals.
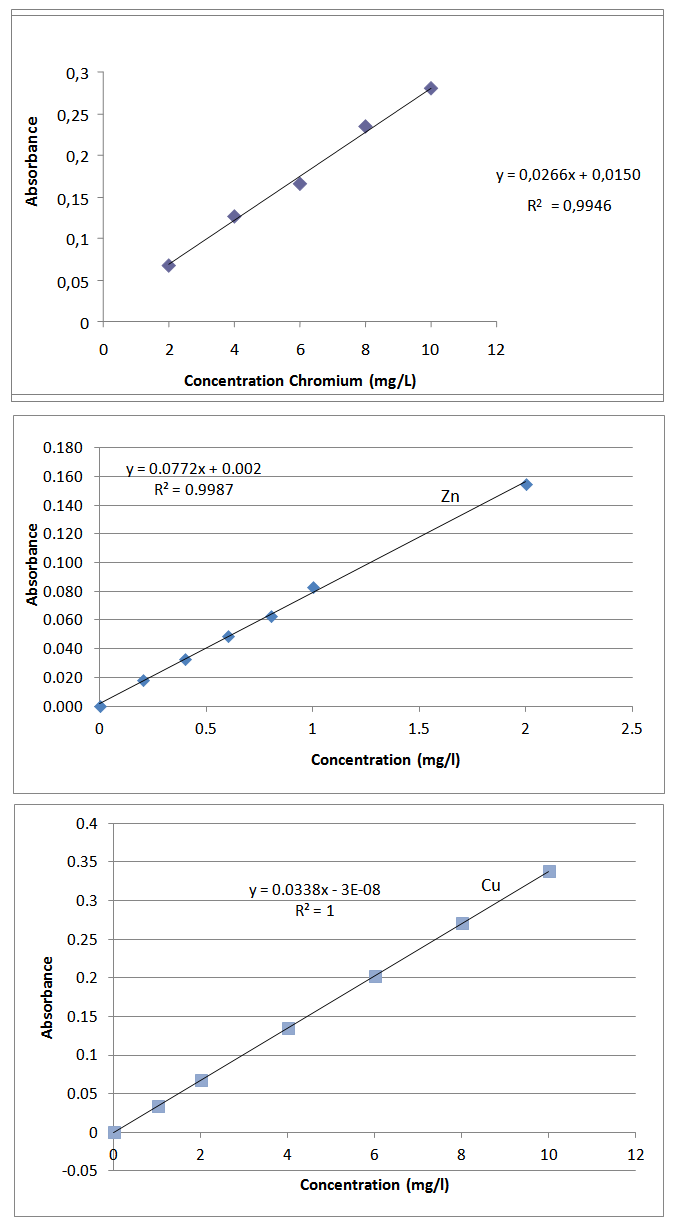 | Calibration graph of chromium, zinc and copper: |
3. Determination of Metals Heavy in Soils
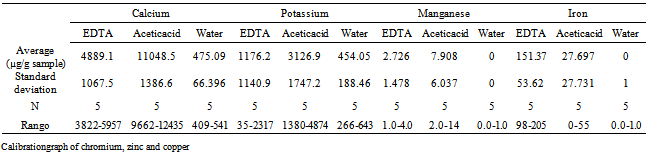
- Using the proposed method, was carried out the study of a sample provided by the University of Zaragoza (Universal Soil fertilized (Auchan)). This is performed with the following three determinations leaching agents: EDTA, HAc and distilled water, so get extract the metals present in the sample and subsequently determine them by EAA and EAA. The results are shown in the following table 4. Depending on the results we can observe that the best leaching agent would be acetic acid (HAc) achieving good results for Ca, Mn and K while for Fe concentration obtained is lower. The second best performing leaching agent offers serious EDTA because the concentrations obtained are significant for all elements studied. Also has the lowest leaching time has over the other leaching agents used, which, provides a significant advantage over the other. Finally, the results indicate that water could be used for analysis of alkaline in our case K and Ca, but other elements such as in the case of Fe and Mn, the results would not be significant.
|
|
|
ACKNOWLEDGMENTS
- The authors thank Prof. Micaela Muñoz from University of Zaragoza for her help. This work was supported by the Department of Science, Technology and University of the Aragon Regional Government and the ESF (group E75) and the University of Zaragoza (proposal #UZ2012-CIE-02).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML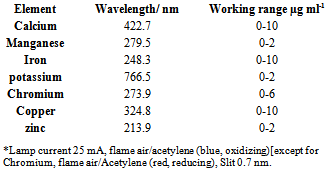
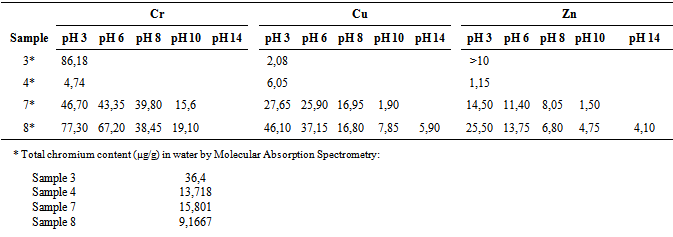 n of chromium, copper and zinc by controlling the pH
n of chromium, copper and zinc by controlling the pH