-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
2013; 1(4): 65-69
doi:10.5923/j.jlce.20130104.02
5E Learning Cycle and the Gas Laws: Constructing and Experimenting with a Ping Pong Popper
Martha M. Day, Matthew Ignash, Lee Ann Smith
GSKyTeach Program, Western Kentucky University, Bowling Green, Kentucky, 42101, USA
Correspondence to: Martha M. Day, GSKyTeach Program, Western Kentucky University, Bowling Green, Kentucky, 42101, USA.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Students interact with the gas laws on a regular basis, but many do not have a deep conceptual understanding of the real world applications of gas laws beyond the boundaries of the classroom. Traditional gas law instruction provides students a theoretical understanding. The authors provide an inquiry-based lesson where students explore the gas laws through the construction and firing of ping pong poppers. Instructional applications include engineering, mathematics, and science as students build and explore the gas laws using the ping pong popper. The authors used the 5E learning cycle (Engage, Explore, Explain, Elaborate, and Evaluate) that encompasses a design activity, thus providing an opportunity for students to apply abstract gas laws to real world applications. By learning about the gas laws through the ping pong poppers, students develop a conceptual understanding of how pressure, temperature, and volume interact.
Keywords: Gas Laws, 5E Inquiry, Charles’ Law, Boyle’s Law, Gay-Lussac’s Law, Constructivist
Cite this paper: Martha M. Day, Matthew Ignash, Lee Ann Smith, 5E Learning Cycle and the Gas Laws: Constructing and Experimenting with a Ping Pong Popper, Journal of Laboratory Chemical Education, Vol. 1 No. 4, 2013, pp. 65-69. doi: 10.5923/j.jlce.20130104.02.
Article Outline
1. Introduction
- The 5E learning cycle is a product of the constructivist learning theory. In a constructivist classroom, the teacher assumes students enter with unique experiences and beliefs about how the world works (i.e. scientific ideas) based upon those experiences[1]. The teacher recognizes changing students’ misconceptions about science is difficult especially since their thoughts are based upon their interpretation of their experiences. Colburn[1] describes how teaching science involves changing students’ minds to help them “understand how and why scientifically accepted explanations explain and predict what will happen in a given situation better than their intuitive ideas.”Bybee, Taylor, Gardner, Van Scotter, Powell, Westbrook, and Landers[2] provided anin-depth discussion of origins, effectiveness, and applications of the 5 Emodel. Wilder and Shuttleworth[3] reported that the effectiveness of thelearning cycle approach has been documented for over forty years.
2. Background
- The 5E learningcycle is an inquiry approach to understanding science concepts. The 5 Es aresuccinctly described:Engagement—captures attention, promotes thinking, raises questions, identifies misconceptions; generate comments, makes connection with prior knowledge.Exploration— poses questions that allow students to test ideas, hypotheses, and alternatives; students make observations, collect data and reach decisions.Explanation— traditional teaching phase; past experiences are used to explain terms and concepts; students use observations and evidence to create and test explanations.Elaboration— deepens understanding by using concepts in new situations; students apply knowledge and skills in a new butsimilar situation.Evaluation—pre, formative, and post assessments occur throughout the learning cycle.
 | Figure 1. The 5E learning cycle starts with engagement and moves clockwise. Evaluation occurs throughout the process |
3. Objectives of Ping Pong Popper and Gas Laws
- 1) Students will construct a model (i.e. ping pong popper) for exploring the properties of gases.2) Students will be able to illustrate how changes in temperature, pressure, and volume affect a system.3) Students will be able to design an experiment to explain the relationships among temperature, volume and pressure with respect to gases.
4. 5E Learning Cycle Procedure
4.1. Engagement
 | Figure 2. the ping pong is launched from the popper |
4.2. Exploration
- Students observe as the instructor demonstrates the ping pong popper (section 6). Instruct the students to examine and observe the construction of the ping pong popper. Ask students to propose an explanation for the event with regard to pressure, volumeand temperature.
4.3. Explanation
- Through probing questioning and prompts, the relationships of pressure, volume, and temperature as related to gases are explained. Examples of probing questions include:1) The ping pong popper most closely relates to which of the engagement examples (e.g. hot air balloon, riding in an airplane, a thermometer, or scuba diver)? Why? 2) Examine the following mathematical formulas (e.g.,
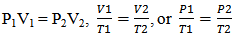 ) used to describe gas laws. Propose a written explanation and illustration to represent the meaning of the mathematical formulas.3) Which formula corresponds to the engagement pictures? Why? For example, with an internal combustion engine, the
) used to describe gas laws. Propose a written explanation and illustration to represent the meaning of the mathematical formulas.3) Which formula corresponds to the engagement pictures? Why? For example, with an internal combustion engine, the 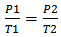 formula most closely corresponds to the visual.4) Rank the mathematical formulas (most to least important) in how they correspond to how the gas interacts in the firing of ping pong popper.5) Propose three or more ways gases are used in our daily lives.
formula most closely corresponds to the visual.4) Rank the mathematical formulas (most to least important) in how they correspond to how the gas interacts in the firing of ping pong popper.5) Propose three or more ways gases are used in our daily lives. 4.4. Elaboration
 | Figure 3. The combustion and expansion of the gas launches the ping pong ball |
4.5. Evaluation
- Formative evaluations exist throughout the 5E learning cycle. For example, during the engagement, an instructor may evaluate students’ prior knowledge. In the exploration stage, students are required to propose explanations for the action of the popper. The explanation stage requires the student to interpret formulas related to the gas laws and the elaboration phase demonstrates that the student can apply the action of the popper to each of the gas laws.Inclusion of an assessment provides students an opportunity to demonstrate their understanding of the gas laws. Assessments may include individual students or student groups presenting their experimental findings to their peers. The authors recommend student presentations that include either traditional scientific poster sessions or multi-media rich talks using PowerPoint, videos, or Prezis.
5. Conclusions
- Constructivist learning theory suggests that humans generate knowledge and meaning through interactions between experiences and ideas. The 5E learning cycle is a proven method for helping students to construct their own understanding of complex science topics. Instructors can help remove the barriers of abstraction that are prevalent in teaching chemistry and make concepts such as the gas laws more accessible through inquiry. If students are allowed ample time and opportunity to consider abstract science topics through inquiry exploration, enduring understanding follows.
6. Ping Pong Popper Assembly
- Overview of Steps1. Punch hole into rubber bulb2. Disassemble sparking mechanism3. Install sparking mechanism into bulb4. Reassemble sparking mechanism5. Test deviceMaterials List for a single ping pong popperButane lighterPing pong ballPlews /Lubrimatic (PLW75-033) Rubber Bulb Type 6in. 6oz. Cap. Battery Filler flint-type spark lantern lighter Small wooden blockPhillip’s head screwdriver or metal punchHammer ProcedureStep 1: Discard the black plastic tube that comes with the battery filler rubber bulb. Slide the wooden block into the rubber bulb. Position the Phillip’s screwdriver as shown and make a hole by striking the screwdriver with a hammer.
 | Figure 4. Punch a hole into the rubber bulb |
 | Figure 5. Loosen the set screw and remove nut with washer |
 | Figure 6. Insert the flint-type spark into the rubber bulb. Pull the spark out of the hole until the thread appears |
 | Figure 7. Constructed ping pong popper |
7. Ping Pong Popper—Engagement
- Students are asked to describe 3 or more properties of gases.Students are then given visuals to examine and reflect upon.a. Which two variables (e.g. temperature, pressure, or volume) are most relevant to the changes that occur in the visual? Why?b. How do those two variables relate to scenario illustrated in the visual? Internal Combustion Engine Example
 a. Temperature and pressureb. As the temperature increased, the pressure inside the combustion chamber increased to the point that a small explosion forced the piston to move outward.Visual 1: A hot air balloon is inflated.Visual 3: A scuba diver descends.
a. Temperature and pressureb. As the temperature increased, the pressure inside the combustion chamber increased to the point that a small explosion forced the piston to move outward.Visual 1: A hot air balloon is inflated.Visual 3: A scuba diver descends.
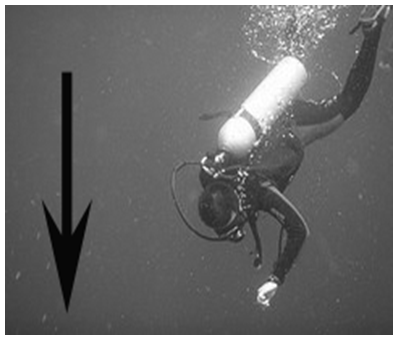 Visual 2: A child’s ears pop as an airplane descends. (You may have experienced your ears popping if you have ever driven up or down a steep hill or mountain.)
Visual 2: A child’s ears pop as an airplane descends. (You may have experienced your ears popping if you have ever driven up or down a steep hill or mountain.)
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML