-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
2013; 1(3): 54-58
doi:10.5923/j.jlce.20130103.04
Simplification of Metal Ion Analysis in Fresh Water Samples by Atomic Absorption Spectroscopy for Laboratory Students
Bhavtosh Sharma1, Shweta Tyagi2
1Uttarakhand Science Education and Research Centre (USERC), Dehradun, 248 006, India
2Department of Chemistry, DAV Post Graduate College, Dehradun, 248 001, India
Correspondence to: Bhavtosh Sharma, Uttarakhand Science Education and Research Centre (USERC), Dehradun, 248 006, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Metal ion analysis is an important aspect during water quality monitoring of either surface or ground water sources. In spite of the availability of several other metal ion analysis techniques for drinking water samples, today atomic absorption spectroscopic (AAS) method is most commonly being used due to the reproducibility of results, short analysis time, cost effective, lower level detection and hyphenated in nature. Therefore, we have attempted to describe the atomic absorption spectroscopic analytical technique in a simple manner. The principle, instrumentation, water sample collection and their preservation, sample preparation, instrument calibration and data analysis for AAS analysis have been described in a simple manner specially for under graduate and post graduate students.
Keywords: Metal Analysis, Fresh Water Sample, Atomic Absorption Spectroscopy
Cite this paper: Bhavtosh Sharma, Shweta Tyagi, Simplification of Metal Ion Analysis in Fresh Water Samples by Atomic Absorption Spectroscopy for Laboratory Students, Journal of Laboratory Chemical Education, Vol. 1 No. 3, 2013, pp. 54-58. doi: 10.5923/j.jlce.20130103.04.
Article Outline
1. Introduction
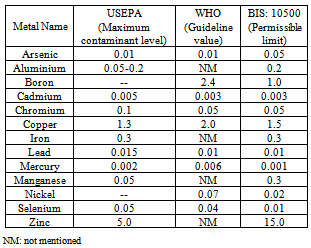
- Water is a principle constituent of the planet earth. Natural sources of fresh water are in the form of rivers, lakes, glaciers and ample ground water system. Metals like aluminium, calcium, cadmium, chromium, copper, iron, lead, magnesium, manganese, zinc etc. may occur in drinking water due to geogenic reasons or may be due to anthropogenic activities such as uncontrolled discharge of waste waters of different types of industries. Besides this, leachates from landfill sites and mining waste dumps are other contributors of metal pollution in drinking water. Some of the metals are necessary to present in drinking water up to desirable limit. But their higher concentrations i.e. more than permissible limit are toxic for human beings. Higher concentrations of these metal ions result in to several types of human health problems[1 - 3]. A comparison of various guide line values of metal ions in drinking water has been made in Table 1[1, 2, 4]. Now a days, several types of detection techniques for metal ions in drinking water samples are available like atomic absorption spectroscopy (AAS), Flame emission, Vapour generation accessory (VGA), Graphite tube atomizer (GTA), Inductively Coupled Plasma Emission, IonChromatography, UV-Visible spectrophotometer, High Performance Liquid Chromatography (HPLC) etc. which are capable to detect the concentration of metal ions up to ppb level[5 - 9]. The speciation of metal ions in drinking water samples can also be achieved by advanced instrumentation techniques including chromatographic, capillary electrophoresis, spectroscopic and other techniques. However, out of these available analytical techniques for metal ion analysis in drinking water samples, AAS method has been selected and described herein due to its wide applicability for metal ion analysis in drinking water samples specially for under graduate, postgraduate students and also for researchers in laboratory.
2. Atomic Absorption Spectroscopy (AAS)
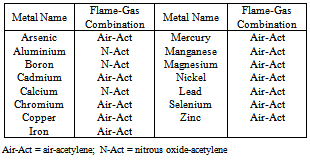
- Atomic Absorption Spectrophotometer analytical instrument is based on the principle of atomic absorption spectroscopy and is very useful to detect the metal ion concentration present in drinking water samples. When a sample solution is aspirated into a flame then sample element is changed into atomic vapour of that element. Flame contains atoms of element. Furthermore, some atoms are thermally excited by flame whereas most of them remain in ground state. The ground state atoms then absorb the radiation of specific wavelength produced by source i.e. hollow cathode lamp of that specific metal. Now, the wavelength of radiation given off by the source or lamp is similar as that of absorbed by the atoms in the flame.
|
|
 | Figure 1. Schematic diagram of an atomic absorption spectrophotometer |
 | Figure 2. An atomic absorption spectrophotometer instrument in the laboratory |
3. Water Sampling and Sample Preservation
- Selection of appropriate sample container is also utmost important to minimize the leaching of contaminants from plastic containers into samples and/or absorption of some sample analytes into the walls of plastic containers. Normally, plastic or glass may be preferred for one material but may be restricted for other. For example, boron, silica and sodium may be leached from soft glass but not plastic. Similarly, trace levels of other metals may be absorbed onto the walls of glass containers. For metal ions, sample containers should be made of high density polyethylene to avoid contamination with metal ions through glass container. But for mercury ion analysis, the samples should be stored in glass container as it reacts with organic materials. Before use, sampling bottles should be rinsed first with tap water, secondly with deionised or ion exchange water and then with nitric acid[2, 10, 12]. A suitable sampling can be performed by using some sampling tools. Manual sampling tools include bacon bombs, dippers, weighted bottles and hand operated pumps. Generally, mechanical, electrical and pneumatic samplers are accessible. Special care should be taken in the selection of the proper instrument for sampling based on the knowledge of the nature of the water being sampled as well as operating conditions. The sampling work should be undertaken according to specified procedures[2, 10]. The samples can be of two types i.e. grab or composite.a. Grab samplesGrab samples are single samples which are collected at a specific spot of a site over a short period of time. Therefore, these samples symbolize only the composition of its source at the time and place of collection. b. Composite samplesComposite samples are collected by taking an appropriate number of grab samples obtained at equal intervals or proportional to flow. Flow proportional composite samples are collected when the flow properties are continually changing. After the completion of sampling task, preservation of the collected water samples is necessary to obtain good results. Therefore, sample preservation can be achieved by making slurry of ice and water for cooling at 4°C to minimize the potential for volatilization or biodegradation between sampling and analysis[10]. Some metal ions like aluminium, cadmium, chromium, copper, iron, lead, manganese, silver and zinc are subject to loss by adsorption on, or ion exchange with the walls of glass containers. Therefore, ultra pure nitric acid is necessary to add into the sample for metal ions preservation. Besides this, acidification of the collected water sample is essential below pH 2.0 to minimize the precipitation and adsorption on container walls.
4. Sample Preparation
- Sample preparation is a very important step in analytical chemistry. Therefore, samples should be prepared very carefully without any mistake or carelessness. The samples for metal ion analysis in drinking water samples by atomic absorption spectrophotometer can be prepared by adopting the standard method as given in scheme 1[2, 10].
5. Preparation of Standard Solution, Standardization, Data Analysis and Calculation
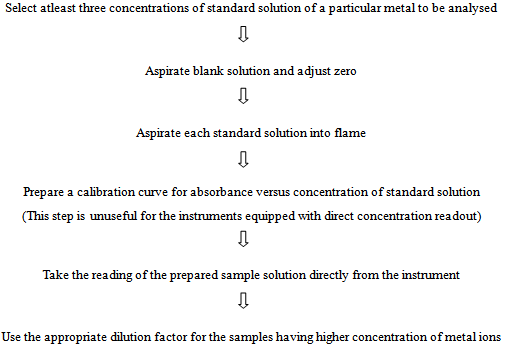
- Standard solutions of metal ions are necessary to standardize and calibrate the instrument. Moreover, the preparation of standards is also an important and crucial part of analysis and it requires much attention of any student or researcher for better results. Therefore, the preparation of standard solution of various metals, standardization, data analysis and calculation can be performed by following the given steps under scheme 2[2, 4, 10].
6. Conclusions
- Atomic absorption spectroscopy has become a method of choice of students and researchers in analytical chemistry due to its wide applicability. The technique is useful not only for metal ion analysis in several matrices like water, soil, sediments, food materials but also for the speciation of metal ions in these matrices. Moreover, hyphenated AAS method has emerged as an essential tool for analytical job. The present article will be helpful for undergraduate, postgraduate and even researchers who possess an interest in laboratory experiments related with analytical chemistry as well as with environmental sciences.
|
|
ACKNOWLEDGEMENTS
- The authors are thankful to the Director USERC, Dehradun and UJS, Dehradun to provide necessary infrastructure and valuable help to this work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML