-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
2013; 1(3): 49-53
doi:10.5923/j.jlce.20130103.03
Using Etching, Electroplating and Lithography as a Laboratory Sequence in Chemistry of Art and Nanotechnology Themed Physical Science Courses
Patrice Bell, Deborah G. Sauder, Julia E. Barker Paredes
School of Science of Technology, Georgia Gwinnett College, Lawrenceville, GA 30043, USA
Correspondence to: Julia E. Barker Paredes, School of Science of Technology, Georgia Gwinnett College, Lawrenceville, GA 30043, USA.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Copper metal may be etched using ferric chloride and subsequently electroplated with zinc to show a variety of chemical concepts for use in non-science major college courses or in high school chemistry courses. The sequence illustrates a variety of chemical concepts applied in Chemistry and Art and Nanotechnology. The reactions may further be used to demonstrate the formation of alloys as well as the utility of lithography.
Keywords: Chemistry of Art, Nanotechnology, Etching, Electroplating, Lithography, Redox
Cite this paper: Patrice Bell, Deborah G. Sauder, Julia E. Barker Paredes, Using Etching, Electroplating and Lithography as a Laboratory Sequence in Chemistry of Art and Nanotechnology Themed Physical Science Courses, Journal of Laboratory Chemical Education, Vol. 1 No. 3, 2013, pp. 49-53. doi: 10.5923/j.jlce.20130103.03.
Article Outline
1. Introduction
- Georgia Gwinnett College (GGC) is a four-year liberal arts institution that fosters use of innovative, active learning strategies coupled with 21st century technology. One of the initiatives within the School of Science and Technology is to develop a more student-engaging science curriculum by introducing themed physical science courses for non-science majors. Themes that faculty have developed and the school has offered include: Chemistry of Art, Nanotechnology, Energy in Society, Food Chemistry, Science of Superheros and Physics for Politicians. Although each of these courses share common course goals including reading charts, texts and graphs as well as understanding the scientific method, each theme applies these goals in different ways as relevant to the particular theme. Students in the physical sciences need one semester of laboratory experience in their two semester physical science sequence. Therefore, each theme with a laboratory component required the development of a complete set (15) of laboratory experiences. As one might imagine, offering a variety of lab themes requires substantial infrastructure to house all the necessary supplies and equipment in addition to requiring faculty and lab personnel time to develop the activities and to prepare all the materials required for each lab session. Therefore, the developers of Chemistry of Art and Nanotechnology laboratory curricula decided to reduce the need for resources by utilizing the procedure described here in courses exploring both the Chemistry of Art and Nanotechnology themes.As the themes have developed, many instructors seem to have a lateral goal of developing a class that is enjoyable and interesting such that non-science majors develop a more positive view of the subject than if they had taken a non-themed course. For example, in conducting the laboratory procedures described in this paper, a student chemically alters a copper sheet and makes their own work of art. Students emboss a pattern on the copper sheet through etching, cause a color change by electroplating the copper with zinc producing a silver color, then form an alloy of the zinc and copper to cause a bronzing effect. Finally, students may use their etched copper sheet as a lithography template and transfer the image to another medium.
1.1. Applicability of Chemistry and Art
- With the popularity of Chemistry and Art courses increasing, there was a distinct interest in making this course very accessible to students while taking advantage of the multitude of instances where the two subjects of Art and Chemistry overlapped.[1-4] Therefore, no book was assigned for this course; only web resources have been utilized. The course at GGC develops concepts of chemistry such as scientific measurements, dimensional analysis, the electromagnetic spectrum, atomic structure and theory, formulas, balancing equations, redox reactions, electroplating, acids and bases, covalent bonding and polymers. Each chemistry topic is illustrated by at least one area of art. For example, when students write formulas and study ionic solids, gemstones are explored. Students see how gemstones are formed in nature and study the crystallized properties of the stones.[5] Several gemstones are used for demonstration both as found in nature and those cut for retail. Students may see the correlation between impurities in crystal structure and inclusions of the gemstone. Other areas of art incorporated into the course include the chemical origins of color, fireworks, photography, paper, polymers/ glue, pottery/ceramics and stained glass. To minimize the impact of not having a textbook, worksheets are employed to keep the course engaging and provide students with resources. A series of “web-outs,” our “green” version of a handout, are also provided to assist students. Though some class material must be delivered through lecture/discussion format, many engaging activities are also employed to introduce chemical concepts. For example, when introducing balancing equations, students are given various colors of molding clay and a worksheet of equations to balance. They are instructed to assign different atoms various colors and demonstrate the balance of equations using the physical representation of the clay. Students are then encouraged to build a sculpture from all of their “atoms” at the end of the exercise. Notably, in developing the Chemistry of Art’s laboratory procedures, there has been a sustained effort to keep all the materials and procedures accessible to anyone, regardless of laboratory availability. Therefore, this opens up the possibility of using the curriculum as a home-schooled laboratory curriculum or in a high school setting. Interestingly, ferric chloride solutions are available through common retailers[6] for use in making circuitry,[7] a factor that facilitated the discussions in nanotechnology applicability. The series of laboratory procedures presented in this paper directly ties into the reduction-oxidation module introduced after the midterm of the Chemistry of Art course. During lecture, students begin to manipulate half reactions resembling those used in early Daguerreotype photography. [8] Several worksheets and “web-outs” are used to demonstrate and provide exercises in the identification of oxidation states, reduction, oxidation, reducing agents, oxidation agents and balancing reduction-oxidation equations. Galvanic and electrochemical cells are discussed and students learn how stored chemical energy is transferred through their use. In the laboratory, students correlate what they have learned in class to how their copper sheet is reacting in the lab, as noted above. Students make their own piece of artwork and may change the color of their copper sheet from copper to metallic silver to bronze. They may also apply the techniques of lithography upon the paper they later make in the laboratory. Therefore, students may generate two unique, separate pieces of art through this sequence of laboratory activities.
1.2. Applicability to Nanotechnology
- In the Nanotechnology themed course, students survey size (nanoscale), fundamentals, tools, and performance of nanostructures, smart materials, sensors, biomedical applications, optics and electronics. To identify tools of nanoscience and ways in which nanostructures are made, students differentiate top-down (silicon integrated circuits) and bottom-up (self-assembly and self-organization) approaches to nanofabrication. There is an assigned textbook [9] for the course. Ratner et al. discuss such nanoscale lithographic examples such as micro/nano-imprint lithography,[10] dip-pen lithography,[11] and nanosphere liftoff lithography.[12] Students also learn about Moore’s Laws 1) indirect, decreasing relationship of the size of an integrated circuit chip and the year that the technology debuts and 2) the opposite, increasing relationship of chip price and debut year.[13] As a result of these studies, students gain a better appreciation for advances in nanotechnology and how the tools used to make nanoscale devices have a great economic impact on society. Students particularly recognize that the decreasing size of computers and their increasing power is associated with smaller, more efficient chips. In addition to learning about nanoscale lithographic examples, nanotechnology students also investigate other fabrication methods involving electroplating and etching processes.In the nanotechnology world, electroplating of surfaces is called electrodeposition in which a nanostructured material may be engineered. For example, a copper sheet of 2-nm thickness may be generated from an electrochemical cell with CuSO4 on the positive electrode and copper will be deposited on a negative titanium electrode.[14] Such engineered nanostructures may have enhanced tensile strength necessary for various technological applications. More advanced fabrication experiments such as those described by Li et al. of the University of Wisconsin- Madison[15] developed nanocomposite micromolds that were fabricated by electroplating onto polymer molds (e.g. LIGA (Lithographie, Galvanoformung, Abformung) processing).[16] Ultimately, Li et al. achieved manufacture of nanocomposites with various hardness by electroplating the composite with nanosized silicon nitride, Si3N4, (< 30-nm).Other approaches to making nanostructures also include electrochemical etching of silicon surfaces in acidic fluoride solutions.[17] This stain etching process can lead to greater control over morphology and properties.In the classroom and laboratory, students study these topics of lithography, electroplating and etching in the context of nanotechnology. Beyond the classroom, students participated in a field trip to the Georgia Institute of Technology Nanotechnology Research Center (NRC) in Atlanta, Georgia. One of the educational and outreach initiatives of the NRC is to educate the public on nanotechnology and to encourage the study of science, technology, engineering and mathematics (STEM) majors. While at the NRC, students participated in hands-on activities that reinforced basic nanotechnology concepts, witnessed nanofabrication and characterization procedures in a cleanroom, and toured several labs with nanoscale probing instrumentation (including scanning electron microscope (SEM) and atomic force microscope (AFM)). Due to the expensive and specialized nature of nanotechnology, most experiments in the Nanotechnology course focus on simple, wet or dry experimental procedures that present macroscopic analogs of the nanotechnology approaches. Additional laboratory periods investigated the metric system, building electronic circuitry and examining Ohm’s Law, and differentiating the appearance of chemical compounds to learn nomenclature. Students developed Excel spread sheets to investigate how the volume to surface ratios of objects change as the objects’ dimensions decrease and to apply the particle in a box model to examine manufacturers reports of the color and size of CdSe or ZnS nanodots. Students separated dye mixtures using paper chromatography, synthesized nanogold particles and magnetic fluids, and examined their chemical and physical properties. This paper presents a set of laboratory exercises that incorporate copper etching, electroplating and lithography that illuminate the concepts taught in both Chemistry of Art and Nanotechnology themed Physical Science courses.
2. Materials
- The materials used for this set experiments include:1. Permanent markers or printed images2. Sheets of copper approximately 3 square inches 0.010 in thick (30 gauge) 3. 20%-40% ferric chloride* aqueous solution (m/m)4. 0.1M zinc chloride aqueous solution5. Strips of Zn metal6. 9 Volt battery with alligator clips and wires7. Bunsen burner 8. Ink pad and/or roller with paper9. Experimental instructions* Danger! Corrosive Material! Make solution slowly as it is quite exothermic upon dissolution.
3. Laboratory Procedure
- Three sequential laboratory procedures are outlined below and may be used in conjunction or separately to demonstrate etching, electroplating/galvanic cells as well as lithography. Depending on the laboratory time available, it would even be possible to combine all three into a single laboratory session.
3.1. Part I: Etching the Copper Sheet
3.1.1. Laboratory Procedure
- After completing an initial pre-lab assignment on laboratory safety and learning how to balance the reduction-oxidation reaction for the experiment (Eq 1-3): [7, 18]
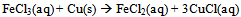 | (1) |
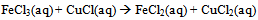 | (2) |
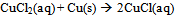 | (3) |
3.1.2. Student Learning Outcomes
- Students determine that a solid metal can be “dissolved” by an aqueous solution of a metal and are introduced to the chemical reality that often many reactions can coincide. Students visually monitor a reaction demonstrating the more difficult concepts of reduction and oxidation. Students are asked to balance each half-reaction, identify what is oxidized/reduced and name the oxidizing/reducing agents for Equations 1-3. They can visually see and physically feel where the copper has been etched and are asked if the areas of the copper that are raised were those that contained ink. Most students can recognize that the areas exposed to the ferric chloride solution were those that underwent a chemical change and were etched. Interestingly, students are quite amazed when they can wash away the Sharpie ink or toner, which lends itself to a fantastic demonstration of physical changes in comparison to chemical changes. Students also gain a respect for working with hazardous materials given the corrosive nature of the ferric chloride solution and the exothermic nature of the process.
3.2. Part II: Electroplating and Forming an Alloy
3.2.1. Laboratory Procedure
- Students may next electroplate their copper sheet by forming a galvanic cell. Typically, the electroplating technique employs Zn2+ (usually Zn(NO3)2 or ZnCl2) and solid strips of Zn, though any silver colored solid metal could be employed. Students are instructed to attach a wire from their copper plate to the (-) anode side of a 9V battery and the (+) cathode of the battery to a sheet of zinc metal. Each of the metal sheets are then placed into a solution of Zn(NO3)2 solution until the desired electroplating consistency is obtained. Students may again use printer toner or a Sharpie pen to coat the areas on their copper sheet they do not wish to be electroplated. After electroplating has occurred, instructors and/or students may then pass the copper sheet over a flame and form an alloy from the combination of the zinc and copper.
3.2.2. Student Learning Outcomes
- Typically, the procedure of electroplating the copper sheets introduced after the common exercise of electroplating a copper penny with zinc and a nickel with copper has been performed.[20] Throughout both of these exercises, students are asked to identify the direction of electron flow and note that electrons are building up on the copper sheet/penny and are thereby attracting Zn2+ ions and forming a small coating of solid Zn on the surface of the copper sheet/penny. Students may also be asked to electroplate a nickel with copper in a similar fashion.Students are tasked with taking the mass of their copper sheet and/or penny before and after electroplating and are often shocked by the apparent decrease in mass of their electroplated item, demonstrating the inherent miniscule nature of the electroplating itself. When perplexed at the source of the decrease of mass, instructors go over to the scale and wave their hand above the balance to demonstrate the sensitivity of the equipment. Students are asked to identify where the oxidation and reduction took place in each situation. They are also asked to predict what would happen if the current of the system was increased.
3.3. Part III: Lithography
3.3.1. Laboratory Procedure
- Lithography is a very simple technique to demonstrate. Simply take an ink pad, preferably with a roller and ink over the etched copper sheet. Then, ask the students to press the inked copper sheet onto a piece of paper. The raised image should transfer to the paper if the copper sheet is flat before the imprinting occurs. (In the Chemistry of Art, students may use paper they construct in lab.)
3.3.2. Student Learning Outcomes
- Students will use their copper sheet to make a lithographic print on paper. The student actually has a visual representation of stamp-assisted lithography process for nanostructures.[21] Microcontact printing is a soft lithographic technique for molecular printing. An elastomeric stamp is inked with a solution of molecules (“ink”) to be printed and brought in contact with a surface with well-defined regions with specific physical and chemical properties.
4. Conclusions
- The series of presented laboratory procedures consisting of etching, electroplating and performing lithography techniques with a sheet of copper may be utilized for several curricula including Nanotechnology and Chemistry of Art themed physical science courses. Students perform procedures demonstrating topics included in both courses including reduction/oxidation reactions and galvanic cells. Furthermore, students make their own artwork through the process and are therefore more engaged with each curriculum. Their artwork also serves as a visual reminder of unseen nanoworld that replicates their experimental bulk process.
ACKNOWLEDGEMENTS
- We would like to thank Georgia Gwinnett College for support of the development of these courses and laboratory procedures.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML