D. Jeiyendira Pradeep, Kapil Dave
Department of Chemical Sciences, Indian Institute of Science Education and Research (IISER) Mohali, Sector-81, Knowledge city, SAS Nagar, Punjab, PO Manauli, 140306, India
Correspondence to: D. Jeiyendira Pradeep, Department of Chemical Sciences, Indian Institute of Science Education and Research (IISER) Mohali, Sector-81, Knowledge city, SAS Nagar, Punjab, PO Manauli, 140306, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Abstract
A new acid-base indicator which is less hazardous and inexpensive is proposed. It is capable of distinguishing between the acids and bases. Titrations were carried out to ensure that the proposed indicator yields quantitative results. The results of titrations were quite satisfactory. The indicator was giving a distinct sustained colour even in the case of weak base and weak acid titrations. Colour of standard indicators like phenolphthalein reappears in titrations even after reaching the end point, but this does not happen in the case of our indicator. Pyrogallol is the chemical which is being used to prepare the indicator solution. Historically, it was heavily used for various purposes like dying and photographic developments. The cost-benefit analysis shows that this indicator is inexpensive when compared with the available standard indicators. Certain indicators have the carcinogenic and flammability issues associated with them, while a study of the MSDS of pyrogallol reveals its less hazardous nature.
Keywords:
Acids, Bases, Indicator, pH
Cite this paper: D. Jeiyendira Pradeep, Kapil Dave, A Novel, Inexpensive and Less Hazardous Acid-Base Indicator, Journal of Laboratory Chemical Education, Vol. 1 No. 2, 2013, pp. 34-38. doi: 10.5923/j.jlce.20130102.04.
1. Introduction
Titrations are the basic chemistry laboratory technique for the quantitative analysis of substances with unknown concentrations using standard solutions of known concentration. The substance with unknown concentration and the standard solution are termed analyte and titrant respectively.Whether it is the task of determining the hardness of water due to calcium and magnesium ions or that of determining the free fatty acid content to be removed from waste vegetable oil to get biodiesel, one can possibly determine the unknown concentration of any chemical substance through titrations.In titrations, the titrant in the calibrated burette is slowly added to a known volume of analyte with a suitable indicator in an Erlenmeyer flask. When there is any change in colour of the analyte solution due to indicator, the titration is complete and the final volume of titrant is noted down using which further calculations are made to find the unknown concentration of analyte. Indicators are used as the markers of the end point of any titrations.The common types of titrations include acid-base titrations, complexometric and redox titrations. In acid-base titration, the unknown concentration of an acid or base is determined by exact neutralization with a base or acid of known. The commonly used indicators in acid-base titrations are shown in Figure 1. ; With the corresponding pH range and colour change.pH indicators are usually weak acids or weak bases which change colour according to the pH of the solution to which it is added. Some of the standard pH indicators are phenolphthalein, methyl orange, methylene blue etc. There are no indicators which have a sharp colour change at one particular pH. The colour change happens over a range of pH, which is different for different indicators. The most commonly used acid-base indicator “litmus” has a wide pH range for colour change. Therefore it is useful for detecting acids and bases over a wide range of pH, whereas phenolphthalein and methyl orange are employed only if the solutions are highly basic or acidic respectively. This is because phenolphthalein has a pH range of 8.3 -10.0 and methyl orange has a pH range of 3.1-4.4 for the colour change. Following reaction scheme describes the working of a pH indicator:-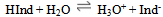 Here HInd stands for the acid form and Ind- for the conjugate base of the indicator. It is the ratio of these two species that determines the colour of the solution. For pH indicators that are weak protolytes, theHenderson-Hasselbalch equation is written as:
Here HInd stands for the acid form and Ind- for the conjugate base of the indicator. It is the ratio of these two species that determines the colour of the solution. For pH indicators that are weak protolytes, theHenderson-Hasselbalch equation is written as: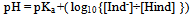
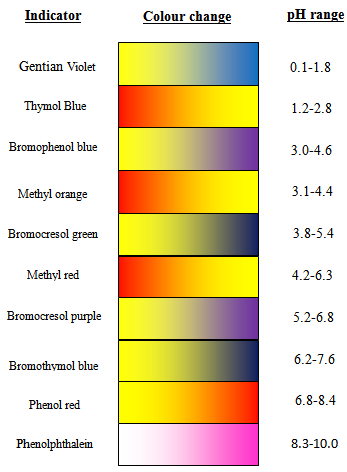 | Figure 1. pH indicator chart |
When pH equals the pKa value of the indicator, both species HInd and Ind- are present in 1:1 ratio. If the pH is above the pKa value, the concentration of the conjugate base is greater than the concentration of the acid, and the color associated with the conjugate base dominates. If the pH is below the pKa value, the reverse happens. This is the theory behind colour transition of indicators in acid-base titrations.Most of the commercially available standard indicators are expensive. In addition to that, they are flammable and toxic too. The hazard risks of the standard indicators are quite evident from the Material Safety Data Sheet[1]. Certain indicators do not show any colour change in weak base versus weak acid titrations. In some cases the colour of the standard indicator like phenolphthalein reappears again and creates confusion in the visual detection of the end point. We have tried to make an acid-base indicator that works well in all acid-base titrations. A study of the structure and mechanism of existing standard indicators like phenolphthalein, methyl orange and methyl red indicated that the colour of the indicator is due to extended conjugation and the presence of OH groups in the compounds. Therefore we chose pyrogallol, (1, 2, 3 benzenetriol Figure 2.), a simple aromatic compound with three OH groups and tested its potential to act as an acid-base indicator.Historically, pyrogallol was used in Hair Dye and as photographic developing agent. Pyrogallol is a white powder and powerful reducing agent. It was first prepared by Scheele by heating gallic acid. It was also used in quantitative analysis of atmospheric oxygen. Its use stopped then due to its suspected its toxicities[2]. In the early 80’s its use was revived. Current studies reveal that pyrogallol is safe for use in cosmetics[3].Pyrogallol is often used to investigate the role of O2·- in biological systems. It has been demonstrated that pyrogallol is an active component of flavonoid for displaying high O2·- scavenging activity because it is much more efficient in scavenging O2·- than catechol[4]. In a recent study, where litchi fruits were treated with pyrogallol at 1 mM and then stored at 25°C or 4°C. Compared with control, pyrogallol significantly reduced pericarp browning and delayed the rotting of fruit on day 4 at 25°C, and on day 30 at 4°C[5].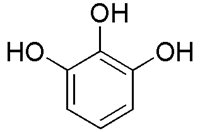 | Figure 2. Chemical Structure of Pyrogallol |
2. Experiment
2.1. Materials and Methods
The chemicals and apparatus used during the study are listed below:-Chemicals Required: Pyrogallol, hydrochloric acid, glacial acetic acid, sodium hydroxide, sodium bicarbonate, and demineralised-water.Apparatus: reagent bottle, weighing balance, spatulas, test tubes, test tube stand, droppers, 50mL burettes, pipettes, pipette filler, funnel, clamp stand, tissue paper, magnetic stirrer, magnetic bead, watch glass, volumetric flasks of 25mL & 50mL, conical flask, pH paper (1.0-12.0), measuring cylinders.
2.2. Preparation of Indicator Solution
About 33-35 mg of pyrogallol (pure) was weighed on a weighing balance and added to 125mL of water in a reagent bottle. The solution was shaken and swirled well, until the entire compound dissolved completely. The indicator is a colourless solution (Figure 3.). Pyrogallol is to be kept in dark bottle and away from any source of heat and light.
2.3. Procedure
At room temperature the following titrations were carried out:a) Strong Acid versus Strong Base: 10mL 0.5 M HCl was titrated with 0.5 M NaOH.b) Weak Acid versus Strong Base: 5mL 0.5 M CH3COOH was titrated with 0.5 M NaOH.c) Strong Acid versus Weak Base: 5mL 0.5 M HCl was titrated with 0.5 M NaHCO3.d) Weak Acid versus Weak Base: 5mL 0.5 M CH3COOH was titrated with 0.5 M NaHCO3.While preparing solutions of the weak base a little heating was given for complete dissolution of the base. When base was titrated with the weak acid, the acid was constantly stirred on a magnetic stirrer. Similarly it was carried out for the other weak bases and weak acids for better results.For titrations involving a weak base our indicator was added in excess (3mL) and for other titrations five drops were added. | Figure 3. Indicator Solution |
3. Results and Discussion
In Table 1 the Colour change of the Indicator in all the above titrations are recorded. In Table 2 the mean volume of base consumed is reported. Table 1. Colour Change of Indicator in Titrations
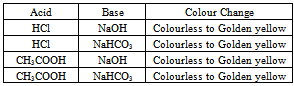 |
| |
|
Table 2. Mean Volume of Base consumed in various titrations
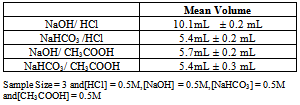 |
| |
|
From Table 2 it is evident that the observed mean values of volume are approximately in good match with the expected theoretical values. Around 30 students used our indicator for titrations and they were satisfied with the performance of the indicator.Addition of our indicator to an acid leads to no colour (Figure 4, Figure 5.), and to base gives a golden yellow colour (Figure 5.). pH range of our indicator is 7.4 - 10.0. Out of the available standard indicators, only phenolphthalein gives distinct colour variation from colourless in acids to pink in bases. Our indicator has a colour transition from colourless to golden yellow (Figure 4.), which is easy to identify.Indicators like phenolphthalein do not show up at all in weak base weak acid titrations[6]. Even if they do, the colour change is short lived.Making a basic golden yellow coloured solution acidic, transforms it into a colourless solution, with very tiny little pale yellowish colour, as in case of methyl orange. The cost of 100 gram pyrogallol is around ₹ 4500 (Currency: Indian Rupees-₹) from Sigma Aldrich as on March 2013. We use only 30 mg pyrogallol in 125mL to prepare the indicator solution. In contrast indicators like phenolphthalein cost around ₹ 3000 for 100 g, and around 2 g of compound is dissolved in 150mL water-ethanol mixture to make the indicator solution. Clearly, the proposed indicator is less expensive.Pyrogallol in lesser concentrations is less toxic and less hazardous. Pyrogallol in large concentrations is toxic to aquatic organisms. It’s harmful for humans only if swallowed[7]. Pyrogallol does not have any carcinogenic effects. Our indicator is made in perhaps the greenest solvent “water”. Pyrogallol can be disposed properly by dissolving or mixing it with a combustible solvent and burnt in a chemical incinerator equipped with an afterburner and scrubber[7].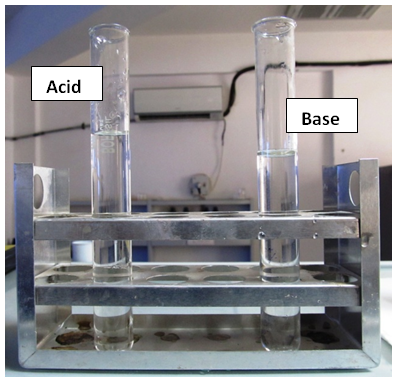 | Figure 4. Acid and Base Solutions (Without Indicator) |
 | Figure 5. Colourless as well as golden yellow coloured acidic and basic solution, respectively after adding the indicator |
The aquatic plant Myriophyllum spicatum produces pyrogallol. The indicator when commercialized, pyrogallol can be synthesized out from the large scale cultivation of this aquatic plant. The possible products after different hydrogen abstractions in pyrogallol are as follows: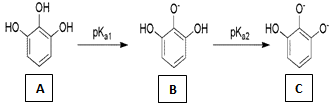 Using the above reaction sequence and the knowledge that pyrogallol is a weak acid; we propose to use the Henderson-Hasselbalch equation to identify the underlying mechanism of colour transition of the indicator[9]. The Henderson-Hasselbalch equation for these different reactions is written as:-
Using the above reaction sequence and the knowledge that pyrogallol is a weak acid; we propose to use the Henderson-Hasselbalch equation to identify the underlying mechanism of colour transition of the indicator[9]. The Henderson-Hasselbalch equation for these different reactions is written as:- 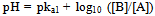 | (1) |
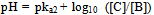 | (2) |
The concentration of A is 0.002 M & the pka values are pKa1= 9.01, pka2 = 11.64[10], where pKa1 refers to abstraction of the 2-hydroxy proton of 1, 2, 3 benzene triol and pka2 refers to the abstraction of either 1 or 3 hydroxy proton of 1, 2, 3 benzene triol. So at pH = 9, Substituting pka1 and[A] in Eq1, gives9 = 9.01 + log10[B] – log[0.002][B] = 0.002 MNow substituting[B] in Eq2. Gives,9 = 11.64 + log10 ([C] /[B])[C] = 0 MSimilarly solving the Henderson-Hasselbalch equation at pH = 10, gives[B] = 0.0195 M &[C] = 0.0004 M, and at pH = 11, gives[B] = 0.1955 M &[C] = 0.0448 M, which again implies the concentration of[B] to be more at pH > 8.From the above calculations, we found that the concentration of[B] is more in the pH range of the indicator, implying that the formation of “B” might be the reason for the change in colour. Pyrogallol also undergoes aerial oxidation in presence of base to give give 3 hydroxy, 1, 2 benzoquinone by electron delocalization within the aromatic ring from the phenoxide ion[11].Some other aromatic alcohols like resorcinol can be investigated for their viability to perform as suitable acid-base indicators.
4. Conclusions
Pyrogallol is a chemical which is in quick access of many laboratories. Also the preparation of this indicator solution is an easy task. Since this indicator has a sustained colour in titrations, students can locate the end point properly. Pyrogallol is less hazardous when compared with carcinogenic and flammable standard indicators. We can use Pyrogallol, the inexpensive and safe chemical as an indicator in acid-base titrations carried out in preliminary chemistry laboratories.
ACKNOWLEDGEMENTS
We thank Professor N. Sathyamurthy (Director IISER Mohali), Dr. Samrat Ghosh (IISER Mohali) and the Department of Chemical Sciences, IISER Mohali for encouraging us and providing us the facilities to carry out our experiments.
References
| [1] | Sigma Aldrich Safety data Sheet, According to Regulation (EC) No. 1907/2006, Version 5.0 Revision Date 30.10.2012 |
| [2] | S. Nakai, Y. Inoue, M. Hosomi and A. Murakami, “Myriophyllum spicatum-released allelopathic polyphenols inhibiting growth of blue-green algae Microcystis aeruginosa” Water Research,34, 3026-3032, 2000. |
| [3] | Final Report on the Safety Assessment of Pyrogallol, International Journal of Toxicology, 10, 67-85, 1991. |
| [4] | Furuno K, Akasako T, Sugihara N: The contribution of the pyrogallol moiety to the superoxide radical scavenging activity of flavonoids. Biol Pharm Bull, 25:19-23. 2002 |
| [5] | G. Jing, H. Huang, Bao Yang, J. Li, Xiaolin Zheng and Yueming Jiang, “Effect of pyrogallol on the physiology and biochemistry of litchi fruit during storage ”, Chemistry Central Journal, 7:19 , 2013 |
| [6] | D. Abugri, O. Apea and G. Pritchett, "Investigation of a Simple and Cheap Source of a Natural Indicator for Acid-Base Titration: Effects of System Conditions on Natural Indicators," Green and Sustainable Chemistry, 2, 117-122, 2012. |
| [7] | Sigma Aldrich Safety data Sheet, According to Regulation (EC) No. 1907/2006 Version 5.1 Revision Date 04.01.2013. |
| [8] | Miyazi K, Arai S, Iwamoto T, Tomoda A, ‘Metabolism of Pyrogallol to purpurogallin by human erythrocytic haemoglobin”, Tohoku Journal of experimental medicine, 203(4):319-30, 2004. |
| [9] | Fundamentals of analytical chemistry, Scoog and West, 368-388. |
| [10] | NTP Technical Report on the Toxiciology and Carcinogenesis studies of pyrogallol, NIH Publication No. 12-5916. |
| [11] | M.K.Mahanti et al. “Kinetics of oxidative coupling of phenols, oxidation of Pyrogallol by alkaline hexacyanoferrate(III)” React. Kinet. Catal. Lett, 22 , 445-449, 1983. |

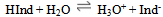 Here HInd stands for the acid form and Ind- for the conjugate base of the indicator. It is the ratio of these two species that determines the colour of the solution. For pH indicators that are weak protolytes, theHenderson-Hasselbalch equation is written as:
Here HInd stands for the acid form and Ind- for the conjugate base of the indicator. It is the ratio of these two species that determines the colour of the solution. For pH indicators that are weak protolytes, theHenderson-Hasselbalch equation is written as: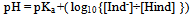





 Using the above reaction sequence and the knowledge that pyrogallol is a weak acid; we propose to use the Henderson-Hasselbalch equation to identify the underlying mechanism of colour transition of the indicator[9]. The Henderson-Hasselbalch equation for these different reactions is written as:-
Using the above reaction sequence and the knowledge that pyrogallol is a weak acid; we propose to use the Henderson-Hasselbalch equation to identify the underlying mechanism of colour transition of the indicator[9]. The Henderson-Hasselbalch equation for these different reactions is written as:- 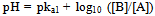
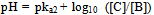
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML
