-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Laboratory Chemical Education
2013; 1(2): 19-24
doi:10.5923/j.jlce.20130102.02
Preparing the Students for the Laboratory by Means of Virtual Labs
José Miguel Rodríguez Mellado1, Manuel Mayén1, Rafael Rodríguez Amaro1, Mercedes Ruiz Montoya2
1Grupo docente UCO 22. Universidad de Córdoba. Campus de Rabanales (Edificio Marie Curie). 14014- Córdoba, Spain
2Grupo docente UCO 22. Universidad de Huelva. Campus de “El Carmen”. E-21071-Huelva, Spain
Correspondence to: José Miguel Rodríguez Mellado, Grupo docente UCO 22. Universidad de Córdoba. Campus de Rabanales (Edificio Marie Curie). 14014- Córdoba, Spain.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
From the teacher viewpoint, this work is useful because the prior training of students who will enter the chemistry lab is done with minimal teacher supervision. This training can be customized for each student and also can be used as part of the assessment of skills in practical classes. The activities allow the student to acquire basic skills and knowledge that will be needed to follow with good use practical classes, this being be done without presence in the classroom.
Keywords: Virtual Laboratories, Educational Software, Basic Laboratory Operations, Simple Experiments
Cite this paper: José Miguel Rodríguez Mellado, Manuel Mayén, Rafael Rodríguez Amaro, Mercedes Ruiz Montoya, Preparing the Students for the Laboratory by Means of Virtual Labs, Journal of Laboratory Chemical Education, Vol. 1 No. 2, 2013, pp. 19-24. doi: 10.5923/j.jlce.20130102.02.
Article Outline
1. Introduction
- Spanish universities are undergoing major changes at the organizational and methodological levels after their joining to European Higher Education Area. One change is connected with the hours for student's personal work, such as those dedicated to the study, literature search, preparation of reports, resolutions of practical cases, preparing seminars or presentations, etc. This implies that, among many other tasks, the student should receive complementary activities from the teacher, and material support. The current university activity increasingly relies more heavily on information technology and communications (ICT), which provide new patterns and new challenges and requirements regarding the education of their members. But there is an increasing volume of information to be processed for conversion into actionable knowledge. They also require a constant improvement of curricula and need constant updating of teachers at the rate of exchange of science and technology. The traditional classroom teaching is, at present, unable to meet the training requirements of the knowledge society, making increasing use most to ICTs.Students in first-degree courses in Spain begin the practical laboratory work with little previous experience. Traditionally, the development of this work begins in the laboratory which provides students with a wealth of new information (recognition and functionality of the material, use appropriate procedures, standard procedures such as distillation, titration etc.) in a relatively short time, which in many cases prevents they assimilate information.The authors have been developing TIC-aided activities and strategies from 2004, being virtual labs presented in part form 2010[1-4].Therefore, the aim of this paper is to present a student activity, based on virtual experiments, which is aimed to prepare the student for the real laboratory.
2. The Goals
- In his facet of laboratory, the objectives of the matter “Chemistry” for first degree courses deal with basic laboratory operations and simple experiments to support the theoretical knowledge. Among basic laboratory operations are:a) To know how to safely use instruments and apparatus commonly used in a chemical laboratory.b) To know the basic safety rules, and to understand the meaning of the labels of chemicals.c) To have knowledge of the organization of space and equipment in a chemical laboratory.d) Knowing and using the basic techniques common in any laboratorye) To be able to manage the waste generated in a chemical laboratory.In addition, simple experiments dealing with thermodynamics and kinetics are usually performed in such first courses.
3. The Virtual Activities
- For each selected activity, specific software, audio-visual material and the corresponding self-evaluation system for the student were developed. In most cases, when the virtual practice is correctly completed, the student gain access to a video of the real experiment. In addition, a report assessing that the student has achieved the practice can be generated to send the teacher for evaluation.It is important to say that the virtual activity is not a “realistic” simulation of the laboratory, but is composed of the calculations and steps to be made in the laboratory, to ensure that the student has understood all the aspects of the practice. The software has been written in Visual Basic.Net, 2010 version.Eleven activities have been developed and integrated in one unique software. The main program has an interface to control the access to each activity. In this program is also included a Periodic Table, developed by one of the authors, which, in addition to the usual information about each element, permits the calculation of molecular weights, this being useful for the development of the experiments.In the main program, a brief introduction of each activity is shown, dealing with the theoretical aspects of the intended experiment/activity. Once the activity is chosen, a new window opens for it.
3.1. Basic Laboratory Operations
- The activity “Basic labware recognition” consists of three phases. In phase 1, as shown in Figure 1, are presented random pics from eight instruments or labware that should be associated with his name, which is selected from a list. No more than two errors are permitted. If the test is not passed, a new collection of photographs is generated and the activity starts again. The student cannot move to the next phase if this phase is not achieved.Phase 2 consists of the random presentation of the descriptions of eight laboratory instruments or labware to be associated with his name, which is selected from a list. No more than two errors are permitted. Again, student cannot move to the next phase if this phase is not achieved.Finally, phase 3 consists of the random presentation of the photographs of five instruments or labware to be associated with their descriptions, selected from a list. No errors are allowed in this phase.The activity “Lab security” consists of the random generation of ten test questions about security selected from a bank of fifty questions. Such questions can be answered as: True, False or Not Answered. For each question it is possible to see an explanation, this feedback permitting the student consolidate its knowledge.
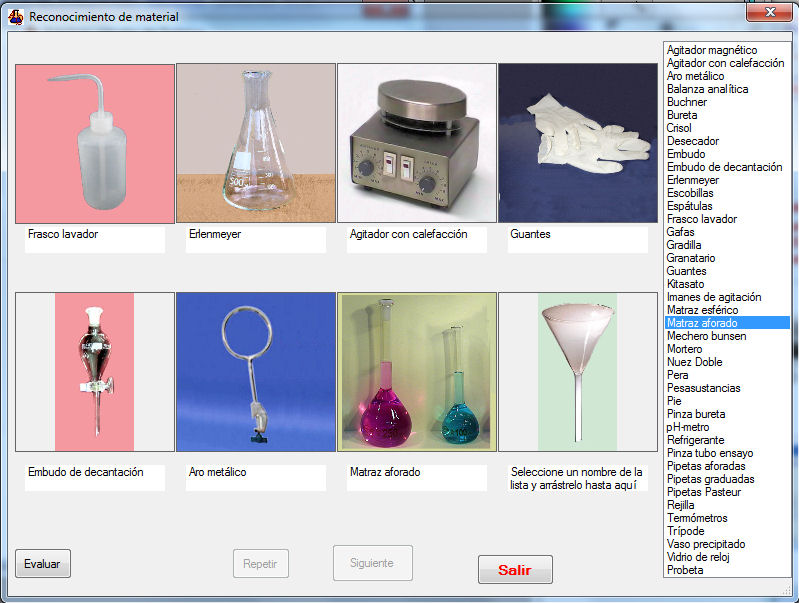 | Figure 1. Basic labware recognition phase 1 |
3.2. Simple Experiments
- The activity “Standardization of NaOH solutions” generates a problem consisting of the preparation of a NaOH solution of a given molarity. First, the student must select the adequate material from a list. Once this task is completed, the operations must be performed in the adequate order. After washing the glassware, the solution must be prepared by weighting the adequate quantity and introduced into a burette. The sodium hydroxide pellets have slightly different weights generated randomly to introduce variability in the weighing. A correct quantity of potassium hydrogenftalate must be introduced into an Erlenmeyer flask and, after addition of fenolftalein, the titration must be made until soft pink colour. If an excess of NaOH is added, an intense pink is observed, as in Figure 2. In this case, the titration can be repeated until the end point is correct. The titration is made twice. From the weights of potassium hydrogenftalate and the volumes of NaOH added, the student must calculate the exact concentration, which will be compared with the generated value.The activity “Oxalic acid adsorption on active carbon” involves the preparation of reactant solutions, after the calculation, which must be correct, the balance of the oxidation-reduction reaction and permanganimetric titrations, as shown in Figure 3. The activity “Catalytic decomposition of hydrogen peroxide” is treated in a similar way.The activity “Surface tension and adsorption” is devoted to illustrate the use of a stalagmometer to establish the relation between surface tension and concentration. From this relation, the parameters of the adsorption of an aliphatic alcohol on the air-water interphase are obtained. The dropping time can be regulated and the general picture of this activity is given in Figure 4.
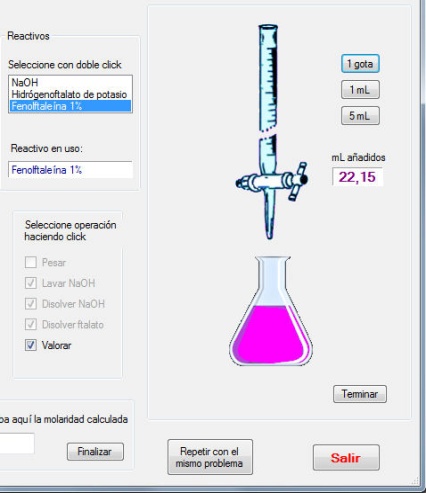 | Figure 2. Detail of NaOH standarization |
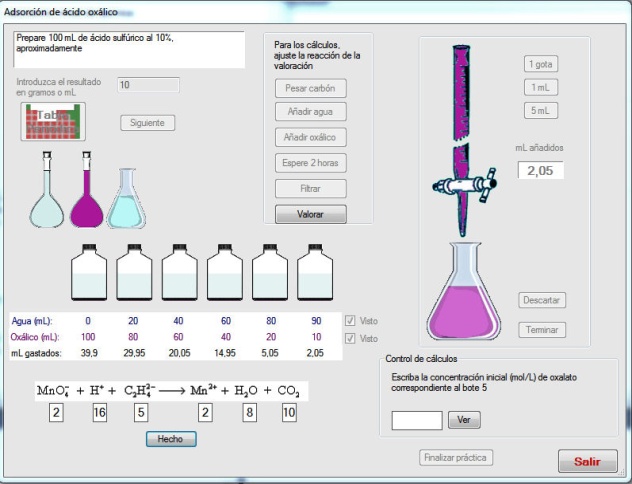 | Figure 3. Adsorption on active carbon |
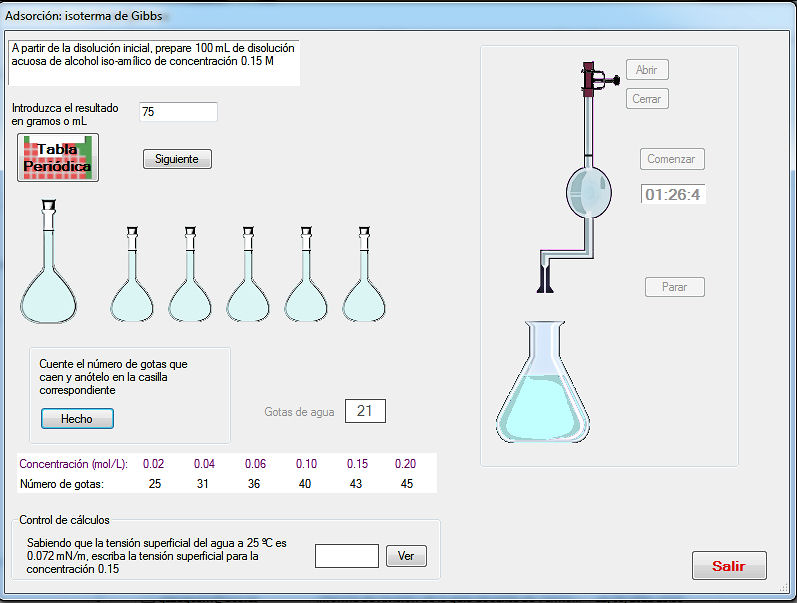 | Figure 4. Adsorption on active carbon |
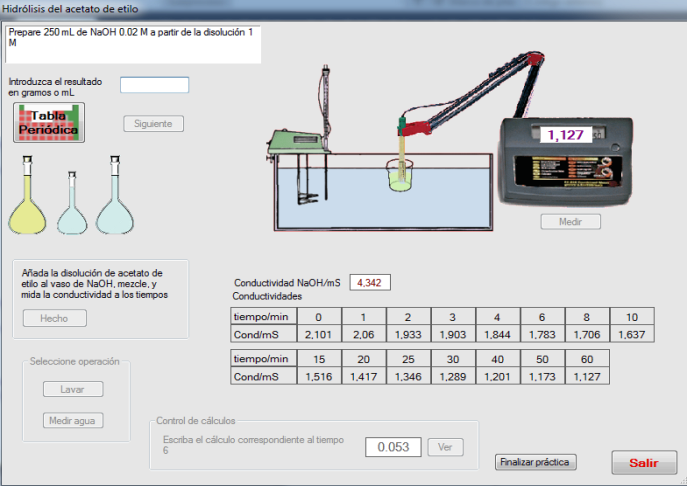 | Figure 5. Kinetics of hydrolysis |
3.3. Usefullness
- These virtual activities have been made available to students prior their access to the real laboratory. A comparison of the development in the laboratory with student of precedent years indicate that, when the virtual lab is used, the students feel more comfortable and less pressed by the teacher, and their work is more fluid.
4. Conclusions
- From the teacher viewpoint, the usefulness of this work lies in the possibility that the prior training of students who will enter the chemistry lab is performed autonomously, with minimal teacher supervision. Moreover it can be customized for each student, so that he can raise concerns and difficulties before the labs. Also it can be used as part of the assessment of skills in practical classes.From the student viewpoint, the software allows one to acquire or strengthen skills, basic skills and knowledge that will be needed to follow with good use practical classes. In addition it can be done independently, without requiring any presentiality, either in the classroom or even in the campus.The software is in process of commercial publication under ISBN, but meanwhile it can be obtained from the authors (e.g. Prof. J. M. Rodríguez-Mellado, e-mail: jmrodriguez@uco.es).
ACKNOWLEDGEMENTS
- To Córdoba and Huelva Universities by the funds provided through their Innovation and Educational Quality Services.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML