-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Civil Engineering Research
p-ISSN: 2163-2316 e-ISSN: 2163-2340
2017; 7(1): 9-16
doi:10.5923/j.jce.20170701.02

Effect of Chlorides and Curing Duration on the Hydration and Strength Development of Plain and Slag Blended Cements
Okiemute Roland Ogirigbo1, Joseph Ukpata2
1Department of Civil Engineering, Faculty of Engineering, University of Benin, Benin City, Nigeria
2Institute for Resilient Infrastructure, School of Civil Engineering, University of Leeds, Leeds, United Kingdom
Correspondence to: Okiemute Roland Ogirigbo, Department of Civil Engineering, Faculty of Engineering, University of Benin, Benin City, Nigeria.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This study investigated the effect of chloride ingress on the hydration mechanism and strength development of plain and slag blended cements. The slag blends were prepared by combining two slags with a CEM I 52.5R-type cement at 30%wt. replacement, while a CEM I 42.5R-type cement was selected as the plain cement. Mortar and paste samples were prepared using a water/binder ratio of 0.5. These were subjected to three different curing regimes – control, 7 and 28 days control curing followed by curing by immersion in a 3% NaCl solution, and tested for compressive strength and hydration using TGA and XRD. Results showed that chloride ingress accelerated the early hydration up to 28 days, and decelerated the later hydration. This effect was more pronounced on samples that were cured for shorter durations before exposure. The slag blends had better strength performance than the plain cement in all curing and exposure conditions.
Keywords: Ground Granulated Blast Furnace Slag, Chloride environment, Compressive strength, Cement hydration, Curing
Cite this paper: Okiemute Roland Ogirigbo, Joseph Ukpata, Effect of Chlorides and Curing Duration on the Hydration and Strength Development of Plain and Slag Blended Cements, Journal of Civil Engineering Research, Vol. 7 No. 1, 2017, pp. 9-16. doi: 10.5923/j.jce.20170701.02.
Article Outline
1. Introduction
- In the construction industry, concrete still remains the most widely used material, and this is mainly because of its good permeability and fire resistance properties, as well as its versatility in that it can easily be moulded into any form or shape [1]. Portland cement, which is a core ingredient in the production of concrete accounts for approximately 7% of anthropogenic global CO2 emissions [2]. This has led to the widespread use of supplementary cementitious materials (SCM) such as fly ash, silica fume, ground granulated blast furnace slag (GGBS), metakaolin, to partially replace Portland cement in the making of concrete.One of these SCMs – GGBS, has been shown to be very beneficial when used as a supplement for Portland cement. Amongst the benefits include: improved workability, higher long-term strength, better thermal performance and resistance to chemical attack from aggressive environments, lower values of chloride diffusion coefficient, and longer corrosion initiation times [3-11]. As a result, several standards now recommend slag blended cements for use in marine construction. For example, CEM IIA/B-S and CEM III A/B/C are Portland slag cements recommended in EN197-1:2011 [12].Concrete structures in marine environments are usually exposed to sulphate and chloride attack, and research has shown that chloride attack is the main initiator of corrosion of the embedded steel reinforcement. According to Shi et al. [13], chloride-induced corrosion of steel reinforcement is the major cause of the early deterioration of marine concrete structures. When chlorides enter into concrete, some of them are bound either physically by the calcium silicate hydrate (C-S-H) phase or chemically by the alumina-ferric oxide-mono sulphate (AFm) phase; while some remain as free chlorides in the pore solution. It is the free chlorides that are responsible for the de-passivation of steel and the lowering of the pH of the concrete surrounding the embedded steel reinforcement, which leads to corrosion [14, 15].Several studies have also shown that curing concrete in chloride environments leads to higher early strength [8, 16] and a decrease in later strength [17]. However, very few studies have looked at the mechanism of how this occurs, especially when considering the ingress of external chlorides and how this differs for plain and slag blended cements. This is very important as it will provide more information on the performance of slag blended cements in marine environments. This study looks at the effect of the ingress of external chlorides on the hydration mechanism of plain and slag blended cements, relating it to the strength development and microstructure.
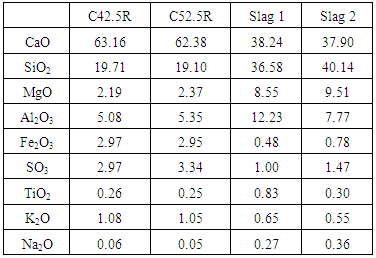
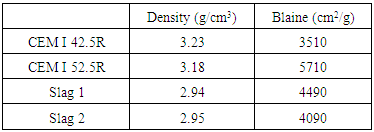
2. Materials and Methods
2.1. Materials
- Two types of Portland cement (CEM I 42.5 R and CEM I 52.5 R) were used for the study. The CEM I 52.5R was replaced partially by 30 per cent by weight of GGBS, while the CEM I 42.5R was used as a reference cement in assessing the performance of the slag blends. Two slags (Slag 1 and Slag 2) with similar physical properties yet different chemical compositions were used for the study. The cements and slags were supplied by a European cement company. Table 1 shows the chemical composition of the cement and the slags as obtained by XRF, while their physical properties are shown in Table 2.
|
|
2.2. Details of Mixes
- A total of three mixes designated as OPC, Slag 1 and Slag 2, were used for the study. The OPC mix represents the mix prepared from the CEM I 42.5R-type cement, while the Slag 1 and Slag 2 mixes represents the mixes prepared from the blending of the CEM I 52.5R-type cement with the two slags (Slag 1 and Slag 2) respectively.
2.3. Preparation of Samples
- Mortar samples were prepared according to EN 196-1:2005 [18]. The samples were prepared by mixing one part of binder with three parts of fine aggregates in a Hobart-type mixer, using a water/binder ratio (w/b) of 0.5. After mixing, the mortar samples were poured into 40 x 40 x 160 mm steel moulds. Cement paste samples were prepared by manual mixing of the cementitious materials and water. After mixing, the resulting paste was poured into 14 mm diameter cylindrical plastic vials.
2.4. Curing Conditions
- Three curing regimes were used for this study: a control, 7 days pre-cured and 28 days pre-cured. These are described as follows:Control: Under this curing regime, the cast mortar samples were covered with thin polythene sheets and left to cure under laboratory air for a period of 20 to 24 hours. After this initial curing, the samples were de-moulded and placed in the curing room at RH of 95% and temperature of 20°C, until the day of testing. For the paste samples, after casting, the top of the plastic vials were sealed with polythene film and allowed to rotate vertically at 20 rpm for 24 hours so as to prevent bleeding. After 24 hours, the paste samples were removed from the plastic vials and cured in saturated lime water at 20°C until testing.7 days pre-cured: Under this curing regime, the cast mortar and paste samples were initially cured under the control curing regime for a period of 7 days, after which they were transferred to tubs containing 3% NaCl solution, where they remained until testing. For the paste samples, the NaCl solution was saturated with lime so as to prevent leaching of calcium hydroxide. This curing regime was selected to replicate marine exposure conditions, where formworks are removed from concrete structures at early ages.28 days pre-cured: This curing regime was similar to the 7 days pre-cured curing regime, except that the samples were cured under the control curing regime for a period of 28 days, before they were transferred to the 3% NaCl solution.
2.5. Test Methods
- Compressive strength was determined in accordance with the procedure outlined in EN 196-1:2005 [18] for mortar samples. Compressive strength was determined at 28 and 90 days. The samples were brought out from the curing tubs at the test date, and cleaned with a dry cloth before testing. Each mortar prism was split into two halves to produce six samples, having an approximate size of 40 x 40 x 80 mm. Thereafter, the split samples were tested for compressive strength using a Tonipact 3000 concrete cube crusher. The compressive strength was taken as the average of six measurements.Thermal analysis was carried out using a Stanton Redcroft 780 series. About 15 to 18 mg of ground hydration stopped cement paste samples were placed in an empty platinum crucible. A corresponding empty platinum crucible was used as the reference. Both the sample and the reference were heated under a nitrogen atmosphere from 20°C to 1000°C at a constant rate of 10°C/min. Hydration was stopped for the cement paste samples using the double solvent exchange technique. Isopropanol was used as the first solvent and was replaced by diethyl ether. Diethyl ether has a lower density than isopropanol and boils at about 35°C, and would therefore displace isopropanol from the pores of the hardened cement paste [19, 20].X-ray diffraction patterns were obtained on hydration stopped cement paste samples that had been ground to particle sizes less than 63 microns. In order to minimise the effect of preferred orientation, care was taken when preparing the samples and minimal pressure was applied when backloading the samples onto the sample holders [21, 22]. Measurements were obtained via a D2 Phaser Bruker diffractometer, using a CuKα radiation in a θ-θ configuration with a fixed divergence split size of 0.5° and a sample stage set to rotate at 15°/min. The samples were scanned over a range of 2θ values (7 to 70°), at a step size of 0.034° and a dwell time of 3 secs.
3. Results and Discussion
3.1. Effect of Chloride Ingress on Compressive Strength
- Figures 1 and 2 shows the effect of chloride ingress on the compressive strength of the plain and the slag blended cements at 28 days and 90 days respectively. From Figure 1, it can be seen that the compressive strength was higher for samples that were pre-cured for 7 days before immersion in the NaCl solution, as compared to those that were cured continuously under water. This implies that chloride ingress accelerated the early strengths of the samples, leading to the higher strengths observed. Similar findings were also reported by [8, 16] and the reason for this is explained in a later section. Comparing the various mixes, it can be seen that the accelerating effect of the chlorides on the strength development was more evident on the slag mixes.At 90 days (Figure 2), the trend was reversed such that samples that were immersed in NaCl solutions had lower strengths than those that were cured continuously under water. This shows the long term deteriorating effect of chlorides on compressive strength as reported previously by [23]. The observed reduction in strength was more significant for the 7 days pre-cured samples as compared to the 28 days pre-cured samples. This shows the benefit of prolonged curing on the strength development of concrete samples exposed to chloride environments, as also observed by [24, 25]. As in the case of the 28 day old samples, the strength loss experienced by the OPC mix was more significant than that of the slag mixes. The reason for this is explained later in Section 3.2.1, and implies that in chloride environments, slag blended cements have better strength performance than plain cements.
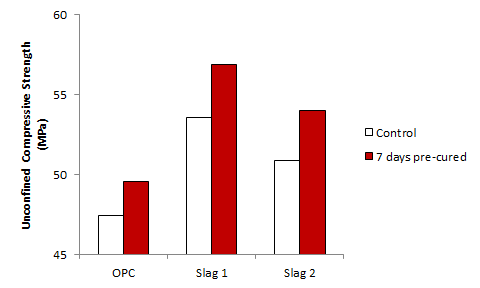 | Figure 1. Effect of chloride ingress on compressive strength development at 28 days |
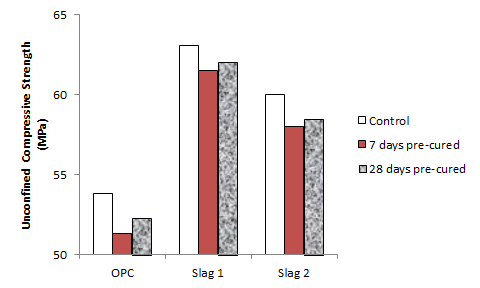 | Figure 2. Effect of chloride ingress on compressive strength development at 90 days |
3.2. Effect of Chloride Ingress on Hydration
3.2.1. Thermal Analysis
- Differential Thermal Gravimetry (DTG) plots obtained from thermal analysis of the cement paste samples after 28 and 90 days of hydration are shown in Figure 3 and 4 respectively. In both figures, the peak ‘FS’ corresponding to Friedel’s salt was observed at a temperature of about 320°C, and was evident only on the samples that were immersed in the NaCl solutions. This indeed confirmed that chloride penetrated into the samples and that chloride binding occurred. Also from both figures, it can be clearly seen that the intensity of the FS peak was greater for the slag mixes than the OPC mix. This implies that more Friedel’s salt was formed in the slag mixes than the OPC mixes. This can be attributed to their higher alumina contents (as seen in Table 1), which will enable them to form more Friedel’s salt [26-29].
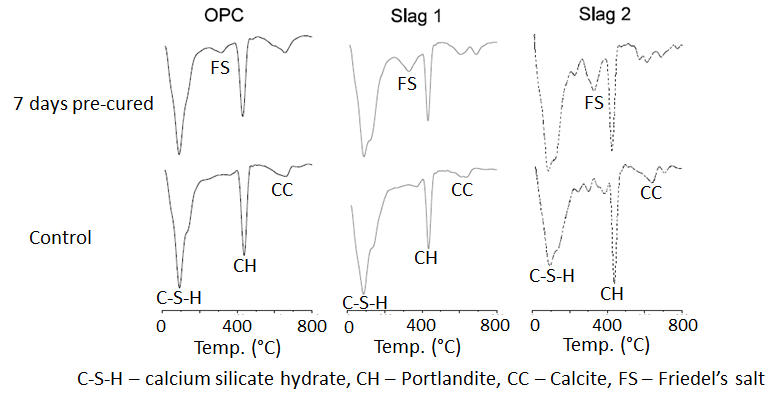 | Figure 3. DTG plots showing the effect of chloride ingress on the hydration products formed after 28 days |
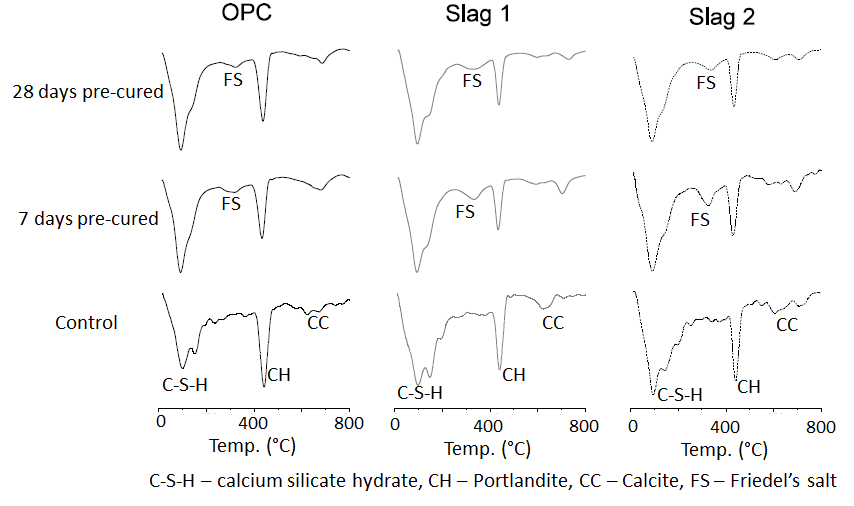 | Figure 4. DTG plots showing the effect of chloride ingress on the hydration products formed after 90 days |
|
3.2.2. X-ray Diffraction Analysis
- Figure 5 and 6 shows the effect of chloride ingress on the hydration of the main clinker phases at 28 and 90 days respectively. As seen in Figure 5, the peaks of C3S, C2S and tri-calcium aluminate (C3A) appear more prominent in the samples that were cured continuously under water as compared to those that were exposed to chloride solutions after 7 days of initial wet curing. This agrees with the DTG plot shown in Figure 3 and indeed confirms that at 28 days, the samples that were pre-cured for 7 days before exposure to chloride solutions had hydrated more than those that were cured continuously under water. However, at 90 days (Figure 6), the trend was seen to reverse, with the peaks of C3S, C2S and C3A appearing more intense for the samples that were exposed to chloride solutions. This explains why the samples that were cured continuously under water had higher strengths at 90 days than those that were exposed to chloride solutions (as seen in Figure 2).
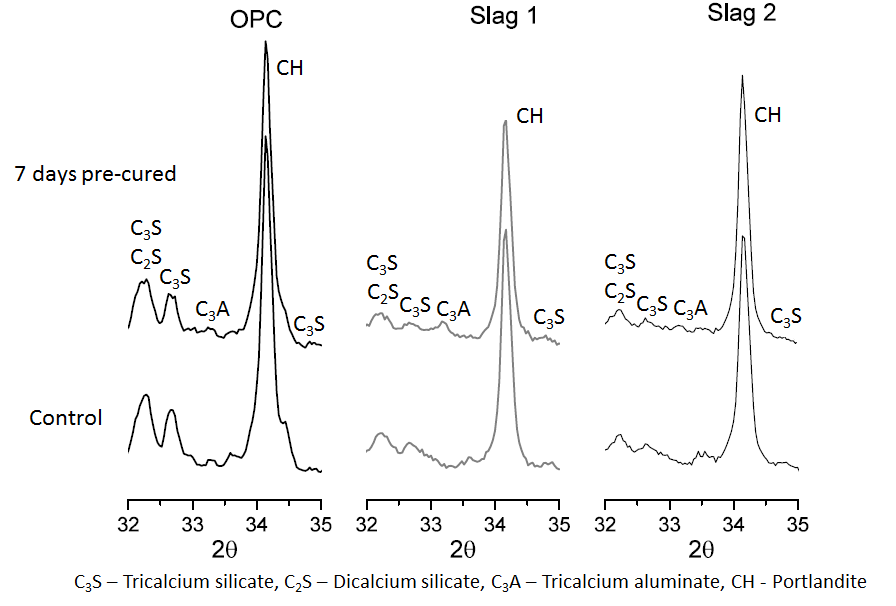 | Figure 5. X-ray diffraction patterns showing the effect of chloride ingress on the hydration of the clinker phases at 28 days |
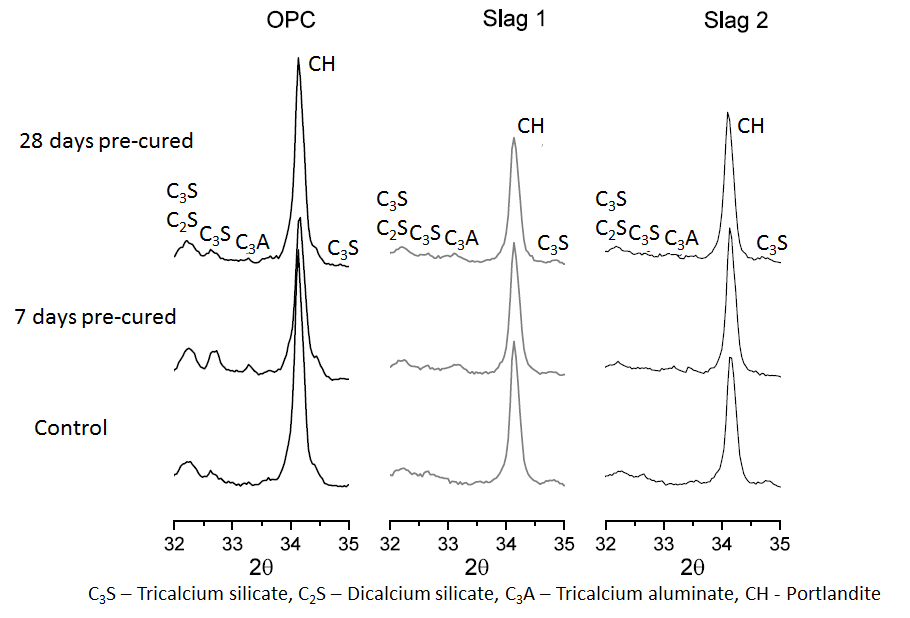 | Figure 6. X-ray diffraction patterns showing the effect of chloride ingress on the hydration of the clinker phases at 90 days |
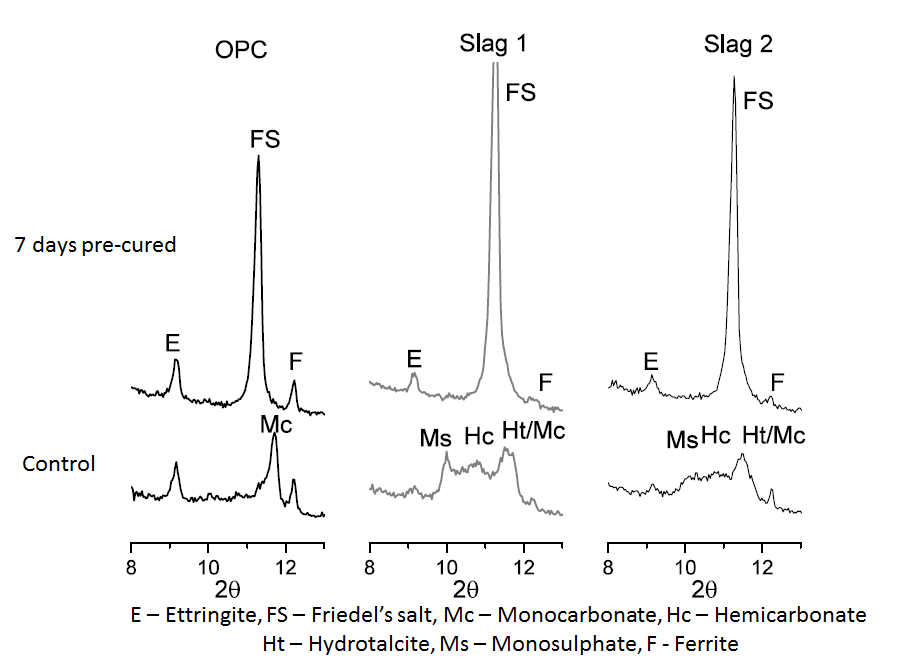 | Figure 7. X-ray diffraction patterns showing the effect of chloride ingress on the formation of the AFm and AFt phases at 28 days |
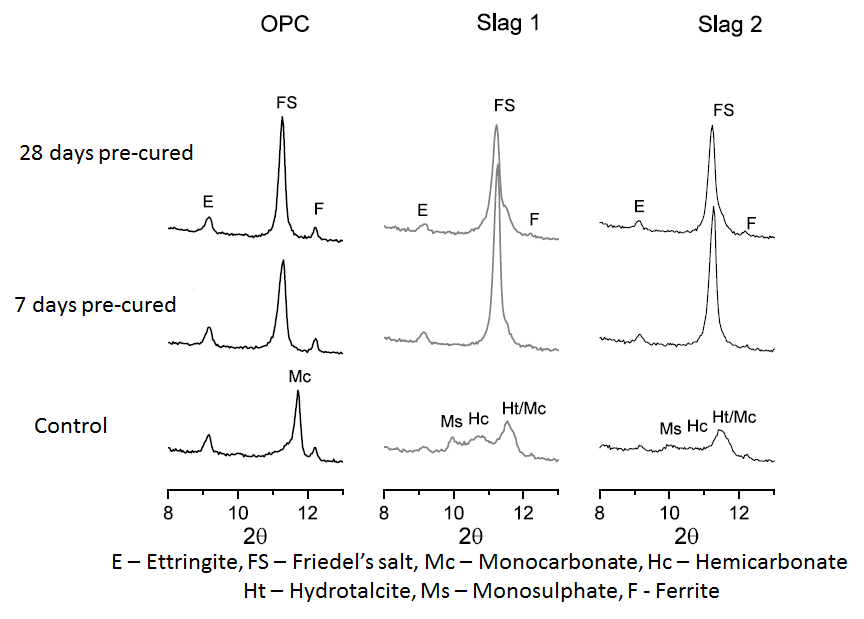 | Figure 8. X-ray diffraction patterns showing the effect of chloride ingress on the formation of the AFm and AFt phases at 90 days |
4. Summary and Conclusions
- This study has investigated the effect of chloride ingress on the hydration and strength development of plain and slag blended cements. From the results obtained, the following conclusions have been drawn:Curing samples in chloride environments can lead to higher early strengths. The results showed that chloride ingress accelerated the early hydration by reacting to form Friedel’s salt that can have a pore blocking effect. This effect was seen to be more pronounced on the slag mixes than the OPC mix, and this was attributed to the higher chloride binding abilities of the slag mixes, enabling them to form more Friedel’s salt.Continuous curing in the chloride solution led to reduction in the later strength at 90 days, this effect was observed to be more significant on the OPC mix than the slag mixes, and also on samples that were water-cured for a shorter period of 7 days before curing in the chloride solution. This was attributed mainly to the higher porosity of the 7 days water-cured samples.Overall, the findings of this study have shown that slag blends have better strength performance than plain cements in chloride environments, and also that prolonged curing can improve the strength performance of concretes exposed to chloride environments.
ACKNOWLEDGEMENTS
- The authors would like to thank Dr. Leon Black of the University of Leeds for his input during the research work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML