-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Instrumentation Science
p-ISSN: 2324-9994 e-ISSN: 2324-9986
2017; 6(1): 8-11
doi:10.5923/j.instrument.20170601.02

Pb(II) Selective Sensor of Poly (vinyl chloride-vinyl acetate)/Polyaniline/Carbon Black
Ayesha Kausar
Nanoscience and Technology Department, National Centre for Physics, Islamabad, Pakistan
Correspondence to: Ayesha Kausar, Nanoscience and Technology Department, National Centre for Physics, Islamabad, Pakistan.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

A new poly(vinyl chloride-vinyl acetate) (PVC-VAc), polyaniline (PANI), and carbon black (CB)-based selective membrane sensor for Pb(II) ions has been prepared using facile route. The potential response of PVC-VAc/PANI and PVC-VAc/PANI/CB membrane sensors as a function of Pb2+ activity was measured. There was improvement in the detection of Pb2+ activity with CB loading. The best response time was obtained for PVC-VAc/PANI/CB 3 sensor with greater CB loading, which was recorded as 10s. The sensors also showed fairly good electrical conductivity up to 10.5 Scm-1. The PVC-VAc/PANI/CB-based sensor has capability to be successfully applied for the direct determination of Pb(II) ions in solution and also as an indicator electrode in potentiometric titration of lead ions.
Keywords: Poly(vinyl chloride-vinyl acetate), Pb(II), Sensor, Conductivity
Cite this paper: Ayesha Kausar, Pb(II) Selective Sensor of Poly (vinyl chloride-vinyl acetate)/Polyaniline/Carbon Black, International Journal of Instrumentation Science, Vol. 6 No. 1, 2017, pp. 8-11. doi: 10.5923/j.instrument.20170601.02.
Article Outline
1. Introduction
- Copolymers of vinyl chloride (VC) and vinyl acetate (VAc) have gained commercial significance and comprehension due to range of exclusive properties [1, 2]. It has been of considerable interest to compare the properties of the copolymer with homopolymers i.e. PVC and PVAc. The properties of PVC and PVAc-based copolymer poly(vinyl chloride-vinyl acetate) (PVC-VAc) have been found intermediate between PVC and PVA [3-6]. Polyaniline, a well known conducting polymer, has been described since several years. It exists in several forms such as aniline black, emeraldine, nigraniline, etc. Polyaniline (PANI) has been synthesized by the chemical or electrochemical oxidation of aniline, (C6H5)NH2 [7-9]. Polyaniline has been synthesized and interconverted in its forms resulting in materials with potentially rich and tailorable chemistry. The novel protonic acid doping of quinoid benzenoid diimine form of polyaniline has been of particular interest. Several studies involve the polyaniline blends with other polymer/copolymers [10-13]. Lead is hazardous omnipresent material in environment. It is a serious poison and tends to accumulate in the bone structure when ingested in levels exceeding the threshold limit. To prevent human safety hazards, it is necessary to monitor lead in the environment due to industrial effluents. To monitor lead and related toxic metals in environmental samples, ion-selective sensors are the convenient means for fast analysis. A number of Pb(II)-selective sensors have been developed and employed to resolve environmental problems [14, 15]. Lead-selective sensors have been prepared using mixture of tetraphenyl borate salt of lead and polyalkoxylate. Lead chelates of naphthalene-1-dithiocarboxylate have also been used in Pb(II)-selective sensors. Moreover, derivatives of porphyrins have been used as electroactive materials in Pb(II)-selective electrodes [16-18]. However, these sensors display narrow working concentration range and suffer interference from metal ions. In this regard electroactive materials of PVC have also been prepared as sensors [19, 20]. The present study involves an investigation on the preparation of poly(vinyl chloride-vinyl acetate), polyaniline, and carbon black (CB)-based Pb2+ selective sensors. Introduction of polyaniline as well as carbon black filler in poly(vinyl chloride-vinyl acetate) matrix led to the successful formation of selective sensors. Such type of lead selective sensor has not been previously reported, so its novel to the best of knowledge.
2. Experimental
2.1. Materials
- Poly(vinyl chloride-vinyl acetate) (PVC-VAc), polyaniline (average Mw ~100,000), carbon black (powder, <10μm), iron(III) chloride anhydrous (≥99.99%), and tetrahydrofuran (THF, anhydrous, 99.9%) were obtained from Aldrich.
2.2. Measurement
- Conductivity measurements were carried out at room temperature using standard four-probe technique. For potential measurements, the prepared membranes were equilibrated for 48 h in 1 M Pb2+ solution. The studies were carried out at 30°C.
2.3. Preparation of Poly(vinyl chloride-vinyl acetate)/polyaniline (PVC-VAc/PANI)
- For the formation of PANI and PVC-VAc blend, 1g PVC-VAc was polymerized with 1g of conductive polyaniline. In-situ polymerization technique was used [21]. THF was used as a solvent. The conductive polymeric matrix (PVC-VAc) was followed by treatment with oxidant solution. FeCl3 was used as an oxidant to form conducting material. After polymerization reaction, the solution was caste in glass Petri’s to obtain film.
2.4. Preparation of poly(vinyl chloride-vinyl acetate)/polyaniline/carbon Black (PVC-VAc/PANI/CB)
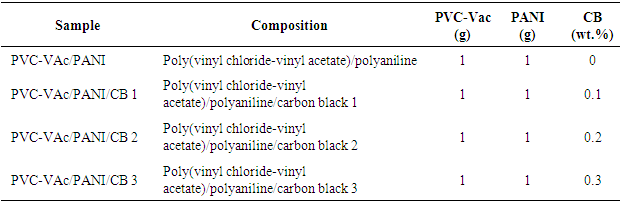
- Different ratios of PVC-VAc, PANI, and CB powders were sonicated for 12 h. The homogeneous mixture obtained were caste in Perti dishes. After casting, the films were dried in vacuum and kept under nitrogen atmosphere [22, 23]. Sample composition used is given in Table 1.
|
3. Results and Discussion
3.1. Potential Measurement
- Saturated calomel electrode (SCE) was used as reference electrode. The cell potentials were measured by varying the concentration of test solution in the range 1.0×10-6 to 1.0×10-1 M by dilution. The pH adjustments were made with HCl/NaOH. The potential response of all the membrane sensors as a function of Pb2+ activity is given in Fig. 1. The response time of the membrane sensors, i.e. the time in which stable and constant potentials are recorded, was determined by measuring the potentials at different time for each sample. It was observed that the PVC-VAc/PANI/CB 3 membrane composition showed significant improvement in the detection of Pb2+ activity. However, the activity was found to be lower for neat PVC-VAc/PANI blend. In short, the prepared sensor membranes were found to be useful in the detection of toxic lead ions.
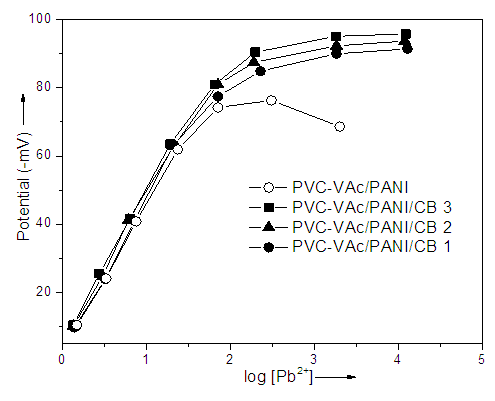 | Figure 1. Variation of cell potential with Pb2+ concentration |
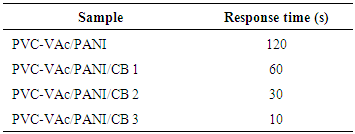
3.2. Response Time
- The response time of the membranes are given in Table 2. The response time of membrane without carbon black filler was found to be approximately 120 s. The addition of CB improved the response time significantly as a sharp reduction in the response time was observed in all the cases. PVC-VAc/PANI/CB 1 membrane performed satisfactorily as the response time was 60s. The best results were obtained for membrane PVC-VAc/PANI/CB 3 sensor, as the response time for this membrane was recorded as 10s. This was the lowest time observed among all the membranes. The results implied that the carbon black content improved the response characteristics of sensor membranes.
|
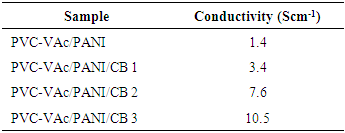
3.3. Conductivity Measurement
- The conductivities of the prepared sensors were found between 1.4-10.5 Scm-1 (Table 3). The conductivity of neat PVC-VAc/PANI was observed low (1.4 Scm-1). However, the conductivity of PVC-VAc/PANI/CB series was found to improve with filler loading. The results depicted that the CB content caused considerable effect on the conductivity of composite sensors. CB is well known conducting nanofiller, and the matrix prepared with polyaniline was also conducting. So, the synergetic effect of polyaniline and carbon black led to the formation of conducting network and improved conductivity. Moreover, the 0.3 wt.% CB composite lose its conductivity slightly in 96 h, as shown in Fig. 2. The properties of novel composites were found better than the other polymer/carbon nanofiller composite reported [24-30].
|
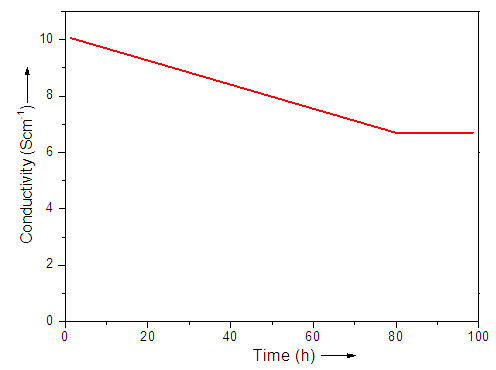 | Figure 2. Conductivity vs. time plot for PVC-VAc/PANI/CB 3 |
4. Conclusions
- Novel poly(vinyl chloride-vinyl acetate), polyaniline, and carbon black based sensor has been successfully prepared and used for the detection of Pb(II) ions. The sensor exhibited good reproducibility and fast response time over a long period of time. The sensor also revealed comparable or better performance to the existing sensors already reported for the determination of lead ions. Moreover, the novel sensor was advantageous in terms of conductivity and environmental stability. Therefore, the proposed sensor can be a good addition to the existing lead ion-selective sensors reported till date.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML