-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Instrumentation Science
p-ISSN: 2324-9994 e-ISSN: 2324-9986
2016; 5(2): 19-23
doi:10.5923/j.instrument.20160502.01

Poly(vinylacetate)cyanomethyl Diphenylcarbamodthioate/ Poly(vinyl acetate)/Carbon Black Composite-based Sensor
Ayesha Kausar
Nanoscience and Technology Department, National Centre for Physics, Quaid-i-Azam University Campus, Islamabad, Pakistan
Correspondence to: Ayesha Kausar, Nanoscience and Technology Department, National Centre for Physics, Quaid-i-Azam University Campus, Islamabad, Pakistan.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In this research attempt, composite of poly(vinylacetate)cyanomethyl diphenylcarbamodithioate (PVACMDT), poly(vinyl acetate) (PVAc) and carbon black (CB) was developed. The poly(vinylacetate)cyano-methyl diphenylcarbamodithioate/poly(vinyl acetate)/carbon black (PVACMDT/PVACMDT/CB) composite were prepared using a facile powder ultrasonication method. The PVACMDT/PVAc/CB composite was tested for gas sensing application. The morphology analysis revealed homogeneous dispersion and network formation of CB particle in the matrix upto 0.5 g. Increase in CB content also enhanced the percolation and conductivity of the composite. In contrast, resisitivity of the sensor was found to decrease in gas vapors. Nevertheless at higher filler content (beyond 0.5 g), resisitivity of the sensor was increased due to aggregation of CB particle.
Keywords: Poly(vinyl acetate), Carbon black, Ultrasonication, Dispersion, Resisitivity
Cite this paper: Ayesha Kausar, Poly(vinylacetate)cyanomethyl Diphenylcarbamodthioate/ Poly(vinyl acetate)/Carbon Black Composite-based Sensor, International Journal of Instrumentation Science, Vol. 5 No. 2, 2016, pp. 19-23. doi: 10.5923/j.instrument.20160502.01.
Article Outline
1. Introduction
- The discharge of gaseous pollutants (oxides of sulfur (SOx) and nitrogen (NOx), toxic gases) from industries has become a severe environmental issue. Analytical gas sensors present a talented solution to hazardous gases in environment. The concentration of gaseous pollutants has been detected and deliberated using sensors [1]. In this regard, conducting polymers have shown potential in sensing gases [2-5]. Moreover, conducting polymer composites are other important potential contenders for sensing gases. Poly(methyl methacrylate) (PMMA), polyvinyl chloride (PVC), polyvinyl acetate (PVAc), and other polymers having functional groups have been utilized to detect gases [6-10]. These conducting composites have been employed for various technical industrial applications. One of the methods for the fabrication of these composite is mixing of conductive particles with insulating polymers, which can modify the final electrical properties. Carbon black (CB) is the most commonly used conducting filler. The polymer/CB composite have been investigated for gas vapor sensing applications [11-14]. It has been demonstrated that the mixing polymer with conducting filler may produce a composition with impermanent electrical conductivity. This effect can also be observed at moderately high temperature [15]. At high temperature, conducting filler particles may form dense chain/network, which allows electrical current to flow. This phenomenon is often termed as percolation [16, 17]. Consequently, percolation point or percolation limit is the point at which the network is fashioned. Generally, the electrical property of polymer/CB composites is the function of morphology [18-20]. Various methods have been reported for the fabrication of polymer/CB composites improving the final material properties [21-23]. Some used physical mixing of CB and the polymer matrix and the composite powders are cross-linked. In-situ polymerization has also been used in the presence of CB. Melt-mixing or solution mixing methods have also been employed [24]. However, there is strong agglomeration tendency in CB particles; therefore melt method is least preferred. The most beneficial methods in this case may be the solution or in-situ polymerization techniques. The ultrasonic treatment has also been used in the formation of homogeneous composite. In this paper, effect of ultrasonic mixing of poly(vinylacetate)cyanomethyl diphenylcarbamodithioate (PVACMDT), poly(vinyl acetate) (PVAc) and CB filler was observed. The poly(vinylacetate)cyano-methyl diphenylcarbamodithioate/ poly(vinyl acetate)/carbon black (PVACMDT/PVACMDT/CB) composite prepared were tested for gas sensing application. The technique was cost effective and facile for the formation of gas detecting composite. Moreover, these composites have been prepared and used for the first time. The novel detector produced was capable of measuring gas concentration in environment.
2. Experimental
2.1. Materials
- Poly(vinyl acetate), cyanomethyl diphenylcarbamodithioate (PVACMDT, average Mn 5,000, PDI <1.2), poly(vinyl acetate) (PVAc, average Mw ~12,800), carbon black (powder, <45μm), and acrylonitrile (99%) were obtained from Aldrich.
2.2. Measurement
- Field Emission Scanning Electron Microscopy (FE-SEM) of freeze fractured samples was performed using JSM5910, JEOL Japan. The electrical resistance was recorded using Hewlett Packard 34401A digital multimeter in four-wire mode.
2.3. Functionalization of Carbon Black
- 2g CB was refluxed in 100 mL of 1M HNO3 at 80°C for 4 h. The product was extracted by centrifugation. The product was washed several times with deionized water till neutral pH. Finally black powder was vacuum dried at 80°C [25].
2.4. Preparation of Poly(vinylacetate)cyanomethyl diphenylcarbamodithioate/poly(vinyl acetate)/ carbon black (PVACMDT/PVAc/CB) Composite
- Different ratio of PVACMDT, PVAc, and CB powders were subjected to ultrasonic mixing for 48 h using ultrasonic mixer. Sample composition used is given in Table 1. The homogeneous mixture obtained was mixed in few drops of acrylonitrile to form a paste (Fig. 1). The PVACMDT/PVAc/CB composite paste was uniformly coated on glass plate to form a gas sensor. The glass plate was fixed on copper electrode to study the conductivity and resistivity.
|
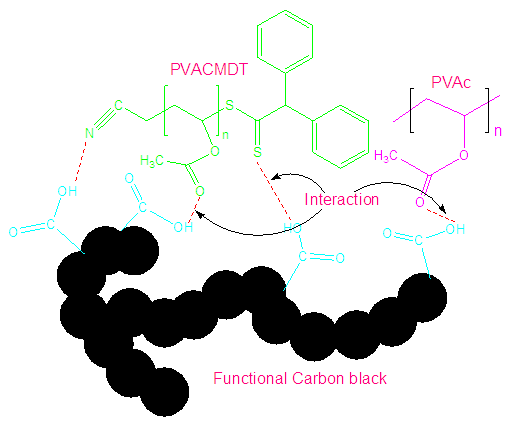 | Figure 1. Formation of PVACMDT/PVAc/CB composite |
3. Results and Discussion
3.1. Morphology STUDY
- The conductivity of composite may decrease if agglomeration of carbon particles occurs in the matrix [26]. However, even dispersion and network formation by carbon black particle may enhance the conductivity of matrix. It can be assumed from the micrographs of PVACMDT/PVAc/CB composite that no agglomerates have developed (Fig. 2). PVACMDT/PVAc/CB 1 revealed fine network formation by polymer coated tiny carbon black particle (Fig. 2A). Increasing the nanofiller content in PVACMDT/PVAc/CB 2 has shown the complex formation with some larger CB particle (Fig. 2B). However, the morphology was considerably altered for the PVACMDT/PVAc/CB 3 composition (Fig. 2C). The network of polymer coated particles was still visible in the micrograph. The over all morphology was uniform with fine dispersion of interconnected CB particle. The morphological development is obviously expected to influence the electrical properties of the composite.
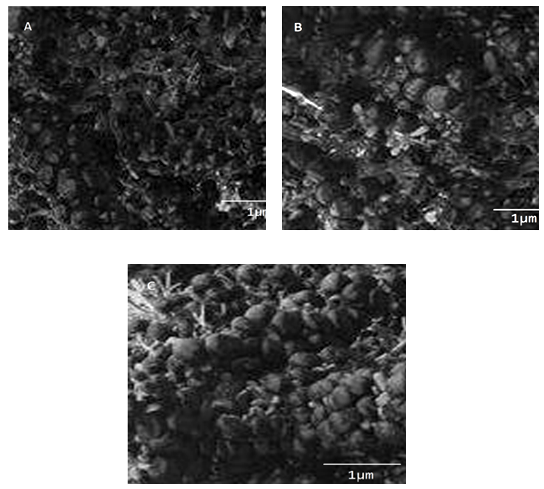 | Figure 2. FESEM micrographs of (A) PVACMDT/PVAc/CB 1; (B) PVACMDT/PVAc/CB 2; and (C) PVACMDT/PVAc/CB 3 |
3.2. Electrical Resistivity of PVACMDT/PVAc/CB Composite
- The relative electrical resistivity response of composite sensor when exposed to acetone vapor is given in Fig. 3. The change in electrical resistance in different sensors was monitored, when the sensing element was exposed to gas [27]. Electrical resistivity has systematically measured for the samples (0.5 h min). Fig. 3 illustrates the resistivity dependence on the functional carbon black content. Obviously, percolation effect occurs in the composite containing higher CB wt.% (PVACMDT/PVAc/CB 3). The resistivity plot exhibits decreasing trend with filler loading. The decrease in resistively was due to continuous network formation by CB particle in polymer phase with increasing temperature. Above 0.5 g CB, continuity of polymer and filler phase was disturbed, thus resistivity was not preserved [28, 29].
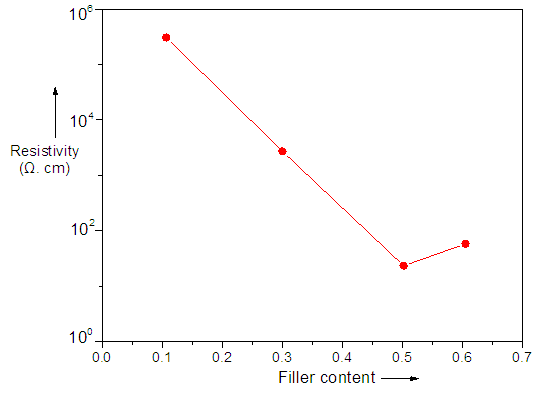 | Figure 3. Dependence of resistivity on CB content |
3.3. Electrical Conductivity of PVACMDT/PVAc/CB Composite
- The relative electrical conductivity response of composite sensor with acetone vapor was also studied. Increasing amount of CB demonstrated increase in percolation threshold of these composite. Fig. 4 shows that the CB dispersion was almost perfectly continuous, even when higher CB content was added. Nevertheless, 0.5g CB was sufficient to form highly continuous phase for the production of electrically conductive composites. In the case of higher filler loading, composite was not adequately conducting due to the aggregation of particulate. Here, percolation threshold as well as conductivity of the composite tends to decrease [30-33].
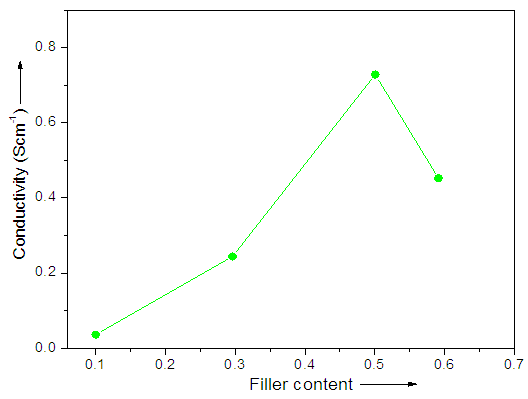 | Figure 4. Dependence of conductivity on CB content |
4. Conclusions
- The ultrasonic mixing was used as an effective method to prepare novel poly(vinylacetate)cyanomethyl diphenylcarbamodithioate, poly(vinyl acetate) and CB-based composite. The material was used to prepare sensing electrode. The FESEM analysis revealed improved homogeneity and network formation. The composite mixture with higher CB content showed improved percolation and conductivity, while reduced resisitivity in electrode. However, beyond certain CB conc. (0.6 g), the percolation and conductivity tend to decrease. The results have shown that PVACMDT/PVAc/CB composite has potential for detecting gas vapors.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML