-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Instrumentation Science
p-ISSN: 2324-9994 e-ISSN: 2324-9986
2016; 5(1): 15-18
doi:10.5923/j.instrument.20160501.03

Design of Polydimethylsiloxane/Nylon 6/Nanodiamond for Sensor Application
Ayesha Kausar
Nanoscience and Technology Department, National Centre For Physics, Quaid-i-Azam University Campus, Islamabad, Pakistan
Correspondence to: Ayesha Kausar, Nanoscience and Technology Department, National Centre For Physics, Quaid-i-Azam University Campus, Islamabad, Pakistan.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In this attempt, polydimethylsiloxane (PDMS) and nylon 6 (Ny 6) were blended as matrix, while nanodiamond (ND) was used as nanofiller for nanocomposite formation. 2,2-Diethoxyacetophenone was used as photo-initiator. Various nanodiamond contents were loaded in PDMS/Ny 6/ND nanocomposite to form the matrix solution. The films were formed on the silicon wafers using dip coating technique. The materials were analyzed for thickness tests, adhesion tests as well as shear modulus G' studies vs. temperature and frequency. Thicknesses 30-45 μm was obtained by applying several layers of nanocomposite on the top of each other for various time spans. Shear modulus was found to be independent of the applied frequency, which was typical for rubber elastic material. However, the shear modulus was found to increase slightly with temperature. Adhesion between nanodiamond and PDMS/Ny 6 matrix was found to be strong because of covalent bonding. Manual peeling of the nanocomposite was not achieved owing to strong adhesion. The novel materials may find potential application in tactile sensors and sensor packaging.
Keywords: Polydimethylsiloxane, Nylon 6, Nanodiamond, Adhesion, Shear modulus, Sensor
Cite this paper: Ayesha Kausar, Design of Polydimethylsiloxane/Nylon 6/Nanodiamond for Sensor Application, International Journal of Instrumentation Science, Vol. 5 No. 1, 2016, pp. 15-18. doi: 10.5923/j.instrument.20160501.03.
Article Outline
1. Introduction
- For modification of polymeric materials pro sensor applications in chemical and biomedical fields, a great deal of research and development effort has been made [1, 2]. By interaction with diverse chemicals, the modulation of material conductivity has been carried out. For detection of organic and inorganic liquid or gases (hydrocarbon, gasoline, ammonia, nitrogen dioxide), sensors have been primarily applied [3, 4]. Generally, suitable sensors are preferred to meet the following features: (i) sensing constituent with high detection rate (ii) reproducible and reversible responses (iii) respond to a wide range of chemicals and concentrations; (iv) easy fabrication with compact, simple and economical design (v) corrosion and weathering resistant [5]. Polydimethylsiloxane (PDMS) is a commercially existing clean room attuned type silicone rubber with extensive range of applications. Generally, it is utilized as mechanical interconnection layer between two silicon wafers (ion selective membranes) and as spring material in accelerometers [6, 7]. Other perspective applications are as top layer elastomers on tactile sensors without effecting device sensitivity. Moreover, they may act as encapsulation material in order to decouple sensors electrically and mechanically from their environment [8, 9]. Because of its low curing temperature, it is also useful in integrated electronics and sensors. In comparison to other polymers, PDMS have [10], low glass transition temperature ~125 °C, shear modulus between 100 kPa and 3 MPa, and low loss tangent tan δ ~0:001. Furthermore, they also have small temperature changes for thermal expansibility α ~20×10-5 K-1 [11, 12], excellent dielectric strength ~ 14 V μm-1, outstanding gas permeability, high compressibility, and usability over a wide temperature range (from -100°C to +100°C) [13]. Although, PDMS have essentially nontoxic nature and have low chemical reactivity (except at extremes of pH). Nylon-6 is a biodegradable and biocompatible polymer. It is extensively used in numerous industrial fields due to its low cost, superior ability of fiber formation, good mechanical strength, and strong chemical and thermal stabilities [14, 15]. Furthermore, electrospun nylon-6 mats have enhanced hydrophilicity and may be a prospective candidate for this application. This polymer is well-recognized with nanoparticles due to its advantages such as hydrophobic features, good stability, UV blocking ability, and excellent photocatalytic and antimicrobacterial capacity [16]. Nanodiamond is well suited due to its outstanding features for numerous applications comprising thermal conducting and heat interface, electronic and optic mechanics, composite electroplating and electroless plating, lubricating oil, and biomedical technology. The high mechanical, optical properties, high surface area, tunable structure, outstanding wear ability, and friction of nanodiamond make them active filler for polymer nanocomposite [17-21]. Nanodiamond films have been deposited by hot filament chemical vapor deposition (HFCVD) method and techniques such as scanning electron microscopy (SEM), Raman spectroscopy and X-Ray diffraction (XRD). The studies have shown effect of carbon concentration on microstructure of nanodiamond films [22-25]. This paper describes the formation and processing of polydimethylsiloxane, nylon 6, and nanodiamond nanocomposite. Various weight content of nanodiamond was loaded in the samples. 2,2-Diethoxyacetophenone was used as photoinitiator. The resulting films were dip coated on the silicon wafers. The layer thickness versus spin rate, and variation of shear modulus G with shear rate were measured and discussed.
2. Experimental
2.1. Materials
- Polydimethylsiloxane, 2,2-diethoxyacetophenone (Photoinitiator, DEAP), and diamond nanopowder were obtained from Aldrich.
2.2. Characterization Techniques
- Thickness of the prepared films was measured with DEKTAK II surface profiler with needle radius 250 μm and stylus force 0.1 mN. The variation in shear modulus G' due to changes in other parameters can be measured with Bohlin rheometer system with frequencies in the range of 0.005 and 30 Hz and temperature between 0° and 80°C.
2.3. Preparation of Polydimethylsiloxane/Nylon 6/Nanodiamond (PDMS/Ny 6/ND) Composite
- To 1 mL of xylene, 50 wt.% of PDMS and 1 wt.% DEAP was added and the solution was sonicated for 6 h at 60°C. 1 wt. % nylon 6 and 1-10 wt.% nanodiamond was dispersed separately in 1 mL of xylene with sonication of 3 h. Later, both the solutions were mixed and centrifuged for 2h at 2000 rpm. The mixture was kept over night for 24 h. The mixture was then coated on silicon wafers using dip casting technique for 1 min. The wafer was rinsed with deionized water and dried at 60°C for 1 h [26].
3. Results and Discussion
3.1. Thickness Study
- The thickness of PDMS/Ny 6/ND film was measured as mentioned in Section 2.2. The measurement results are shown in Fig. 1. Thicknesses about 30-45 μm was obtained by applying several layers of nanocomposite on the top of each other. The results have shown that the thickness of both PDMS/Ny 6/ND 5 (5 wt.% nanodiamond) and PDMS/Ny 6/ND 10 (10 wt.% nanodiamond) nanocomposite was found to increase with the dipping time. The maximum results were obtained for the PDMS/Ny 6/ND 10 nanocomposite having increased nanodiamond content.
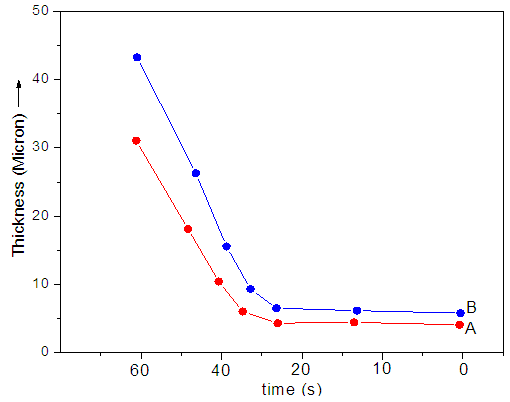 | Figure 1. Thickness at different coating time (A) PDMS/Ny 6/ND 5 and (B) PDMS/Ny 6/ND 10 |
3.2. Shear Modulus Versus Frequency
- The shear elastic modulus G can be divided into real and imaginary part [27]. The variation in shear modulus G' due to changes in frequency was measured with Bohlin rheometer system. The shear modulus G' versus applied frequency is shown in Fig. 2. Generally, shear modulus is independent of the applied frequency, which is typical for a rubber elastic material. The results reinforce the applicability of the material for sensor application.
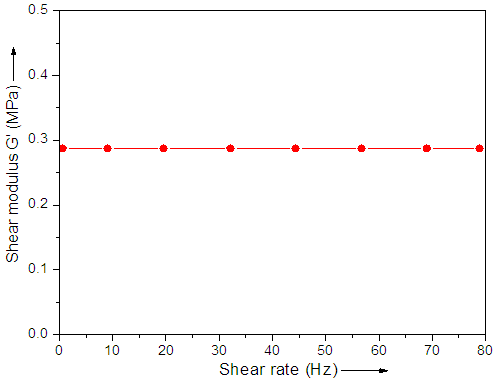 | Figure 2. Shear modulus G' versus shear rate |
3.3. Shear Modulus Versus Temperature
- The shear modulus was seemed to be independent of the applied temperature, as shown in Fig. 3. The figure shows that the shear modulus increased with the temperature, which is typical for rubber elastic materials [28]. However, the change in shear modulus G' was so small that the value could not be determined with precision. The results indicated that the change in shear modulus G' was almost steady for the analyzed samples.
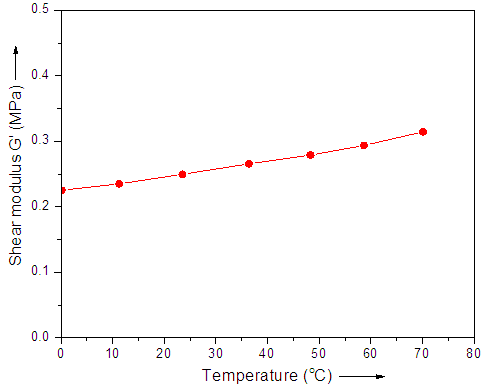 | Figure 3. Shear modulus G' versus temperature |
3.4. Adhesion of PDMS/Ny 6/ND Nanocomposite
- Nanodiamond acted as coupling agent between the PDMS/Ny 6 polymers and the silicon substrate. The nanocomposite was capable of chemical interaction with the silicon oxide surface. In this way, nanodiamond acted as a linker to bind the silicon oxide surface to PDMS/Ny 6 matrix covalently [29]. The adhesion between the nanocomposite and substrate was very strong. Manual peel test was applied to separate the nanocomposite from the wafer; the material was not found to separate from the wafers. The results depicted strapping linkage between the filler and matrices due to effective interfacial contact.
4. Conclusions
- According to the results, PDMS/Ny 6/ND nanocomposite showed rubber elastic behavior. The shear modulus was found to be independent of the applied frequency. However, it showed small change with temperature. Because of the nanodiamond as PDMS/Ny 6/ND component, it showed fine adhesion to the silicon substrate. Results have suggested that the novel PDMS/Ny 6/ND nanocomposite materials have shown several potential applications in tangible sensors and also in packaging for sensor.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML