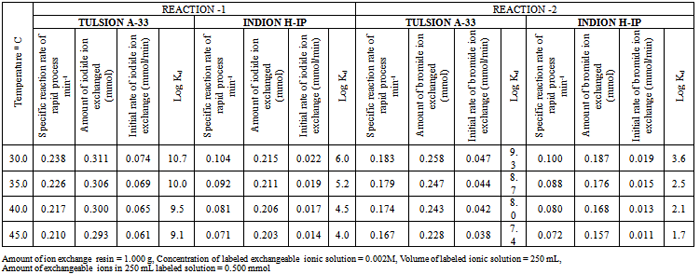
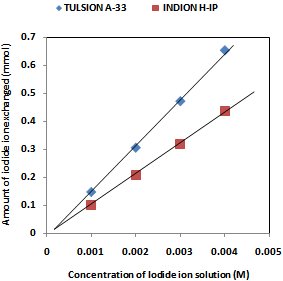
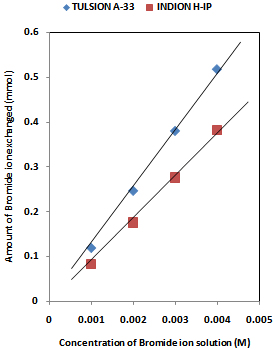
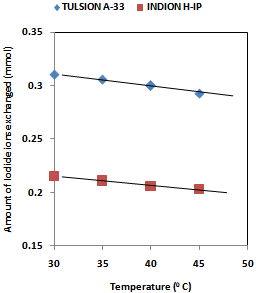
| [1] | Hou, X., Celler, A., Grimes, J., Benard, F., and Ruth, T., 2012, Theoretical dosimetry estimations for radioisotopes produced by proton-induced reactions on natural and enriched molybdenum targets., Physics in Medicine and Biology, 57(6), 1499-1515. |
| [2] | Sadeghi, M., Enferadi, M., and Nadi, H., 2010, Study of the cyclotron production of 172Lu: An excellent radiotracer., J. Radioanalytical and Nuclear Chemistry, 286(1), 259-263. |
| [3] | Milton, B.F., and Stevenson, N.R., 1995, Cyclotrons for isotope production., Proceedings of the IEEE Particle Accelerator Conference, Dallas, TX, USA, 1, 89-91. |
| [4] | Sood, D.D., Reddy, A.V.R., and Ramamurthy, N., 2004, Applications of Radioisotopes In Physico-Chemical Investigations, in Fundamentals of Radiochemistry, Indian Association of Nuclear Chemists and Allied Scientists (IANCAS), 253-263. |
| [5] | Koron, N., Bratkic, A., Ribeiro Guevara, S., Vahcic, M., and Horvat, M., 2012, Mercury methylation and reduction potentials in marine water: An improved methodology using 197Hg radiotracer., Applied Radiation and Isotopes, 70(1), 46-50. |
| [6] | Bartsch, A., Zollmer, V., Ratzke, K., Meyer, A., and Faupel, F., 2011, Diffusion and viscous flow in bulk glass forming alloys, J. Alloys and Compounds, 509(Supplement 1), S2-S7. |
| [7] | Gibson, N., Holzwarth, U., Abbas, K., Simonelli, F., Kozempel, J., Cydzik, I., Cotogno, G., Bulgheroni, A., Gilliland, D., Ponti, J., Franchini, F., Marmorato, P., Stamm, H., Kreyling, W., Wenk, A., Semmler-Behnke, M., Buono, S., MacIocco, L., and Burgio, N., 2011, Radiolabelling of engineered nanoparticles for in vitro and in vivo tracing applications using cyclotron accelerators., Archives of Toxicology, 85(7), 751-773. |
| [8] | Sanchez-Cabeza, J.A., Levy, I., Gastaud, J., Eriksson, M., Osvath, I., Aoyama, M., Povinec, P.P., and Komura, K., 2011, Transport of North Pacific 137Cs labeled waters to the south- eastern Atlantic Ocean., Progress in Oceanography, 89(1-4), 31-37. |
| [9] | Venugopal, V., 2011, Societal applications of nuclear technology in health care, industry and water resource management in India, Energy Procedia., 2nd International Conference on Asian Nuclear Prospects (ANUP 2010) Mamallapuram; India, 7, 553-559. |
| [10] | Ghitescu, P., and Ghizdeanu, N.B., 2008, Evaluation of the possibility of plutonium and minor actinides transmutation in HWR., International Conference on Advances in Nuclear Power Plants (ICAPP 2008), Anaheim, CA, 4, 2051-2058. |
| [11] | El Mamoney, M.H., and Ashraf Khater, E.M., 2004, Environmental characterization and radio-ecological impacts of non-nuclear industries on the Red Sea coast., J. Environmental Radioactivity, 73(2), 151–168. |
| [12] | Gaillot, S., Guigon, A., Cathaumj, S., Parrat, D., and Bayon, G., 2006, In-pool neutron raliography for the jules horowitz reactor: A key non destructive equipmental process., 8th World Conference on Neutron Radiography, WCNR-8;Gaithersburg, MD. |
| [13] | Radiotracer Applications in Industry- A Guidebook, 2004, Safety Reports Series No. 423, International Atomic Energy Agency (IAEA) Vienna. |
| [14] | Murakami, K. Yu, X., Kato, T., Inoue, Y., and Sugawara, K., 2012, Synthesis of temperature- responsive anion exchanger via click reaction., Journal of Colloid and Interface Science, 376(1), 189-195. |
| [15] | Zhang, F., Li, Y., Guo, Z., Liang, T., Yang, B., Zhou, Y., and Liang, X., 2011, A polar-copolymerized method to prepare silica-based anion exchanger for ion chromatography., Talanta,85(1), 112-116. |
| [16] | Chen, S., Yue, Q., Gao, B., Li, Q., and Xu, X., 2011, Preparation and characteristics of anion exchanger from corn stalks., Desalination, 274(1-3), 113-119. |
| [17] | Sood, D.D., 1998, Proc. Int. Conf. on Applications of Radioisotopes and Radiation in Industrial Development, ed. Sood, D.D., Reddy, A.V.R., Iyer, S.R.K., Gangadharan, S., and Singh, G., (B.A.R.C., India) 35–53. |
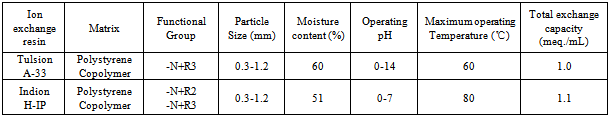
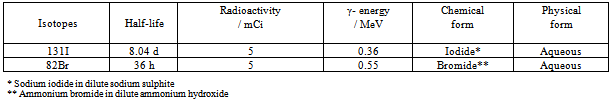
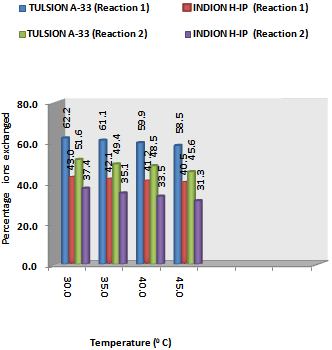
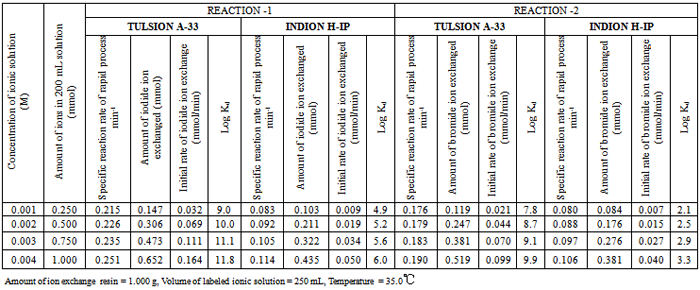
| [18] | Singare, P.U., and Lokhande, R.S., 2012, Studies on Ion-Isotopic Exchange Reactions Using Nuclear Grade Ion Exchange Resins., Ionics, 18(4), 351–357. |
| [19] | Lokhande, R.S., Singare, P.U., and Kolte, A.R., 2010, Application of Radioactive Tracer Technique for Characterization of Strongly Basic Anion Exchange Resins Duolite A 101D and Duolite A 102D., Radiochemistry, 52(1), 81–86. |
| [20] | Lokhande, R.S., Singare, P.U., Tiwari, S.R.D., 2009, Application of 82Br as a Radioactive Tracer Isotope to Study the Bromide Ion-Isotopic Exchange Reaction in Strongly Basic Anion Exchange Resin Duolite – A161., Russ. J.Physical Chemistry, 83(8), 1389-1394. |
| [21] | Lokhande, R.S, Singare, P.U., and Tiwari, S.R.D., 2008, Study of Bromide Ion- Isotopic Exchange Reaction Kinetics Using a weakly Basic Macro porous Resin Indion – 860., Radiochemistry, 50(6), 633-637. |
| [22] | Lokhande, R.S., Singare, P.U., and Parab, S.A., 2008, Application of Radioactive Tracer Technique to Study the Kinetics of Iodide Ion- Isotopic Exchange Reaction using Strongly Basic Anion Exchange Resin Duolite A-116., Radiochemistry, 50(6), 642-644. |
| [23] | Lokhande, R.S., Singare, P.U., and Patil, V.V., 2008, Application of Radioactive Tracer Technique to Study the Kinetics and Mechanism of Reversible Ion- Isotopic Exchange Reaction using Strongly Basic Anion Exchange Resin Indion -850., Radiochemistry,50(6), 638-641. |
| [24] | Lokhande, R.S., Singare, P.U., and Prabhavalkar, T.S., 2008, The Application of the Radioactive Tracer Technique to Study the Kinetics of Bromide Isotope Exchange Reaction with the Participation of Strongly Basic Anion Exchange Resin Indion FF-IP., Russ. J. Physical Chemistry A, 82(9), 1589–1595. |
| [25] | Singare, P.U., Lokhande, R.S., and Patil, A.B., 2008, Application of Radioactive Tracer Technique for Characterization of some Strongly Basic Anion Exchange Resins., Radiochim. Acta, 96(2), 99-104. |
| [26] | Lokhande, R.S., and Singare, P.U., 2008, Comparative Study on Iodide and Bromide Ion-Isotopic Exchange Reactions by Application of Radioactive Tracer Technique., J.Porous Mater., 15(3), 253-258. |
| [27] | Lokhande, R.S., Singare, P.U., and Patil, A.B., 2007, Application of Radioactive Tracer Technique on Industrial Grade Ion Exchange Resins Indion-830 (Type-1) and Indion-N-IP (Type-2)., Radiochim. Acta, 95(1), 111-114. |
| [28] | Lokhande, R.S., and Singare, P.U., 2007, Comparative Study on Ion-Isotopic Exchange Reaction Kinetics by Application of Tracer Technique., Radiochim. Acta, 95(3), 173-176. |
| [29] | Lokhande, R.S., Singare, P.U., and Kolte, A.R., 2007, Study on Kinetics and Mechanism of Ion-Isotopic Exchange Reaction Using Strongly Basic Anion Exchange Resins Duolite A-101 D and Duolite A-102 D., Radiochim. Acta, 95(10), 595-600. |
| [30] | Lokhande, R.S., Singare, P.U., and Dole, M.H., 2007, Application of Radiotracer Technique to Study the Ion Isotope Exchange Reactions Using a Strongly Basic Anion-Exchange Resin Duolite A-113., Radiochemistry, 49(5), 519-522. |
| [31] | Lokhande, R.S., Singare, P.U., and Karthikeyan, P., 2007, The Kinetics and Mechanism of Bromide Ion Isotope Exchange Reaction in Strongly Basic Anion-Exchange Resin Duolite A-162 Determined by the Radioactive Tracer Technique., Russ. J. Physical Chemistry A, 81(11), 1768–1773. |
| [32] | Lokhande, R.S., Singare, P.U., and Dole, M.H., 2006, Comparative Study on Bromide and Iodide Ion-Isotopic Exchange Reactions Using Strongly Basic Anion Exchange Resin Duolite A-113., J. Nuclear and Radiochemical Sciences, 7(2), 29-32. |
| [33] | Lokhande, R.S., and Singare, P.U., 2003, Study of reversible ion-isotopic self diffusion reaction using 82 Br as a radioactive tracer isotope., Asian J. Chem., 15(1), 33-37. |
| [34] | Lokhande, R.S., and Singare, P.U., 2005, Study on kinetics of self diffusion reaction by application of 82 Br as a radioactive tracer isotope., Asian J. Chem., 17(1), 125-128. |
| [35] | Shannon, R.D., 1976, Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides., Acta Crystallographica, A32, 751-767. |
| [36] | Heumann, K.G., and Baier, K., 1982, Chloride distribution coefficient on strongly basic anion- exchange resin: Dependence on co-ion in alkali fluoride solutions., Chromatographia, 15(11), 701-703. |
| [37] | Adachi, S., Mizuno, T., and Matsuno, R., 1995, Concentration dependence of the distribution coefficient of maltooligosaccharides on a cation-exchange resin., J. Chromatography A, 708(2), 177-183. |
| [38] | Shuji, A., Takcshi, M., and Ryuichi, M., 1996, Temperature dependence of the distribution coefficient of maltooligosaccharides on cation-exchange resin in Na+ form., Bioscience, Biotechnology, and Biochemistry, 60(2), 338-340. |



 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML