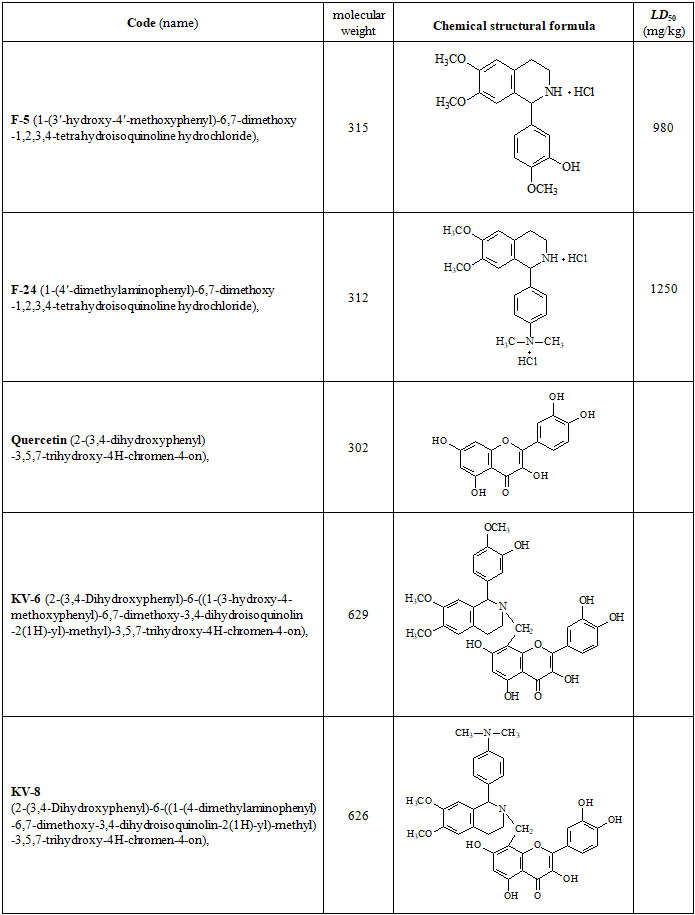
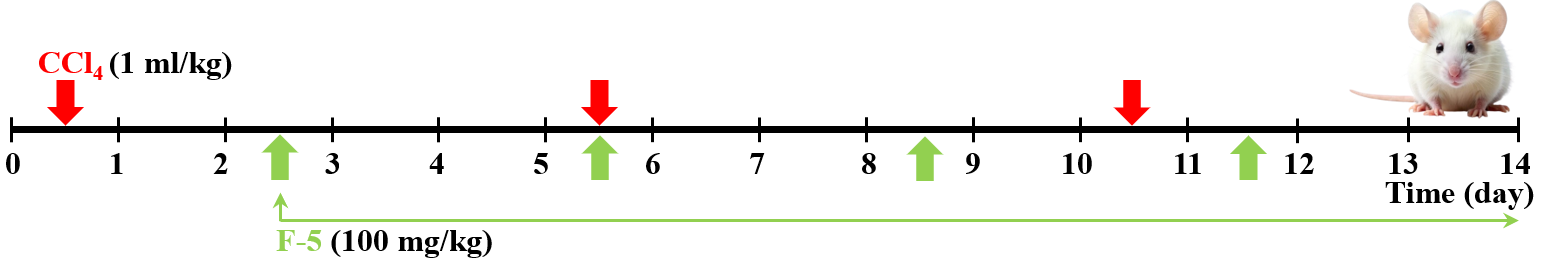
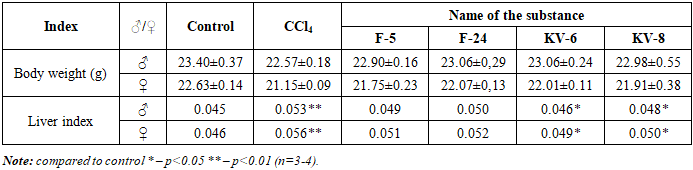
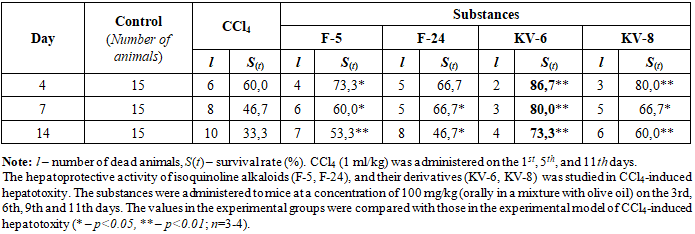
| [1] | Abdulkhakova G.V., Komilov E.Dzh., Ergashev N.A., Asrarov M.I., Makhmudov R.R., Kenzhaeva M.F. Effect of tannins hexagalloylglucose and heptagalloylglucose on ATP-dependent potassium channels in vitro // “Universum: Chemistry and Biology” (Electronic Scientific Journal). – 2025. – №5(131). – P.59-64. (In Russian). |
| [2] | Abrashova T.V., Gushchin Ya.A., Kovaleva M.A., Rybakova A.V., Selezneva A.I., Sokolova A.P., Khodko S.V. Physiological, biochemical and biometric indicators of the norm of experimental animals (Handbook) (Edited by: Prof. Makarova V.G., Makarova M.N.) // St. Petersburg: Publishing house “LEMA”, 2013. – 116 p. |
| [3] | Aditi P., Ali V., Choubey M., Tirumalasetty M.B., Pandey H., Srivastava S., Tripathi Y.B. Hepatoprotective role of Pueraria tuberosa water extract (PTWE) in CCl4-induced liver injury through different signaling pathways // Advances in Traditional Medicine. – 2014. – P.1-16. |
| [4] | Algefare A.I., Alfwuaires M., Famurewa A.C., Elsawy H., Sedky A. Geraniol prevents CCl4-induced hepatotoxicity via suppression of hepatic oxidative stress, pro-inflammation and apoptosis in rats // Toxicology Reports. – 2024. – V.12. – P.128-134. |
| [5] | Alhassan A.J., Sule M.S., Aliyu S.A., Aliyu M.D. Ideal hepatotoxicity model in rats using carbon tetrachloride (CCl4) // Bayero Journal of Pure and Applied Sciences. – 2009. – V.2(2). – P.185-187. |
| [6] | Ali A.A. Biochemical and Histological Study of Rat Liver Injury Induced by carbon tetrachloride (CCl4) // Wasit Journal for Pure Sciences. – 2025. – V.4(1). – P.156-167. |
| [7] | Anijdan S.H.M., Mahdavi S.R., Shirazi A., Zarrinfard M.A., Hajati J. Megavoltage X-ray dose enhancement with gold nanoparticles in tumor bearing mice // IJMCM. – 2013. – V.2(3). – P.118-124. |
| [8] | Azamatov A.A., Zhurakulov S.N., Vinogradova V.I., Tursunkhodzhaeva F., Khinkar R.M., Malatani R.T., Aldurdunji M.M., Tiezzi A., Mamadalieva N.Z. Evaluation of the local anesthetic activity, acute toxicity, and structure-toxicity relationship in series of synthesized 1-aryltetrahydroisoquinoline alkaloid derivatives in vivo and in silico // Molecules 2023, 28, 477). – P.1-17. |
| [9] | Begum R., Papia S.A., Begum M.M., Wang H., Karim R., Sultana R., Das P.R., Begum T., Islam R., Manwar N., Rahman S. Evaluation of hepatoprotective potential of polyherbal preparations in CCl4-induced hepatotoxicity in mice // Advances in Pharmacological and Pharmaceutical Sciences. – 2022(3169500). – P.1-9. |
| [10] | Belyaev A. M., Mikhnin A. E., Rogachev M. V. Data preparation and survival analysis in the MedCalc and Statistica statistical software packages (Study guide for students in the system of higher and additional professional education) // St. Petersburg (N.N. Petrov National Medical Research Center of Oncology), 2022. – 56 p. (In Russian). |
| [11] | Besednova N. N., Zaporozhets T. S., Kuznetsova T. A., Kryzhanovsky S. P., Kovalev N. N., Zvyagintseva T. N. Hepatoprotective effects of seaweed extracts and polysaccharides // Antibiotics and chemotherapy. – 2014. – #59(3-4). – P.30-37. (In Russian). |
| [12] | Bishnolia M., Yadav P., Singh S.K., Manhar N., Rajput S., Khurana A., Bhatti K.S., Navik U. Methyl donor ameliorates CCl4-induced liver fibrosis by inhibiting inflammation, and fibrosis through the downregulation of EGFR and DNMT-1 expression // Food and Chemical Toxicology. – 2025. – V.196(115230). – P.1-8. |
| [13] | Bocharov E. V., Karpova R. V., Bocharova O. A., Kucheryanu V. G., Shprakh Z. S. The effect of a multiphytoadaptogen in early postnatal ontogenesis, improving the survival and somatic state of mice of a high-cancer line // Russian Journal of Biotherapeutics. – 2017. – #.16. – P.76-81. (In Russian). |
| [14] | Bueverov A.O. Possibilities of treating drug-induced liver injury in conditions of the need to take hepatotoxic drugs // The attending physician. – 2009. – #.2. – P.40-42. (In Russian). |
| [15] | Byun J.-H., Kim J., Choung S.-Y. Hepaprotective effect of standardized Ecklonia stolonifera formulation on CCl4-induced liver injury in Sprague-Dawley rats // Biomol. Ther. – 2018. – V.26(2). – P.218-223. |
| [16] | Chang M.-L., Yeh C.-T., Chang P.-Y., Chen J.-C. Comparison of murine cirrhosis models induced by hepatotoxin administration and common bile duct ligation // World J. Gastroenterol. – 2005. – V.11(27). – P.4167-4172. |
| [17] | Chen P., Zhou Y.-K., Han C.-S., Chen L.-J., Wang Y.-M., Zhuang Z.-M., Lin S., Zhou Y.-H., Jiang J.-H., Yang R.-L. Stem cells from human exfoliated deciduous teeth alleviate liver cirrhosis via inhibition of gasdermin D-executed hepatocyte pyroptosis // Front. Immunol. – 2022. – V.13(860225). – Р.1-12. |
| [18] | Chen Y., Li R., Hu N., Yu C., Song H., Li Y., Dai Y., Guo Z., Li M., Zheng Y., Guo Z., Qi Y. Baihe Wuyao decoction ameliorates CCl4-induced chronic liver injury and liver fibrosis in mice through blocking TGF-β1/Smad2/3 signaling, anti-inflammation and anti-oxidation effects // Journal of Ethnopharmacology. – 2020. – V.263(113227). – P.1-11. |
| [19] | Das M., Boerma M., Goree J.R., Lavoie E.G., Fausther M., Gubrij I.B., Pangle A.K., Johnson L.G., Dranoff J.A. Pathological changes in pulmonary circulation in carbon tetrachloride (CCl4)-induced cirrhotic mice // PLoS ONE. – 2014. – V.9(4): e96043. – P.1-9. |
| [20] | Delgado-Montemayor C., Cordero-Perez P., Salazar-Aranda R., Waksman-Minskya N. Models of hepatoprotective activity assessment // Medicina Universitaria. – 2015. – V.17(69). – P.222-228. |
| [21] | Dong M., Yang P., Zhang X., Nie S., Sun X. Nrf2 deficiency brings about increased sensitive to IR and 7,12-dimethylbenz(a) anthracene and leukemia predisposition // Dose-Response: An International Journal. – 2025. – V.23(2). – P.1-11. |
| [22] | Dospekhov B.A. Methodology of field experiment (With the basics of statistical processing of research results) // 5th edition, Moscow. – “Agropromizdat Publishing House”, 1985. – P.347-415. (In Russian). |
| [23] | Elshafey S.M.A., Abdelrahman A.A., Tukhbatova R.I., Ivanova E.V., Akinina E.A., Voronkova Yu.E., Bukuru L.K., Fattakhova A.N., Alimova F.K. The influence of Nigella sativa and Salvia officinalis vegetable oils on the biochemical parameters of CD-1 mice // Scientific notes of Kazan University. – 2013. – T.155(3). – P.82-89. (In Russian). |
| [24] | Ernst L., Zieglowski L., Schulz M., Moss M., Meyer M.,Weiskirchen R., Palme R., Hamann M., Talbot S.R., Tolba R.H. Severity assessment in mice subjected to carbon tetrachloride // Scientific Reports. – 2020. – V.10(15790). – P.1-11. |
| [25] | Fortea J., Fernandez-Mena C., Puerto M., Ripoll C., Almagro J., Banares J., Bellon J.M., Banares R., Vaquero J. Comparison of two protocols of carbon tetrachloride-induced cirrhosis in rats – improving yield and reproducibility // Scientific Reports. – 2018. – V.8(9163). – P.1-10. |
| [26] | Fu X., Jiang B., Zheng B., Yan Y., Wang J., Duan Y., Li S., Yan L., Wang H., Chen B., Sang X., Ji W., Xu R.-H., Si W. Heterogenic transplantation of bone marrow-derived rhesus macaque mesenchymal stem cells ameliorates liver fibrosis induced by carbon tetrachloride in mouse // PeerJ. – 2018. – V.6(e4336). – P.1-23. |
| [27] | Fujii T., Fuchs B.C., Yamada S., Lauwers G.Y., Kulu Y., Goodwin J.M., Lanuti M., Tanabe K.K. Research article mouse model of carbon tetrachloride induced liver fibrosis: Histopathological changes and expression of CD133 and epidermal growth factor // BMC Gastroenterology 2010. – V.10(79). – P.1-11. |
| [28] | Gan C., Yuan Y., Shen H., Gao J., Kong X., Che Z., Guo Y., Wang H., Dong E., Xiao J. Liver diseases: Epidemiology, causes, trends and predictions // Signal Transduction and Targeted Therapy. – 2025. – V.10(33). – P.1-36. |
| [29] | Ghosh A., Sil P. Protection of acetaminophen induced mitochondrial dysfunctions and hepatic necrosis via Akt-NF-kappaB pathway: Role of a novel protein // Chem. Biol. Interact. – 2009. – V.177. – P.96-106. |
| [30] | Glanz S. Medical and Biological Statistics // Moscow. – “Praktika Publishing House”. – 1999. – P.250-459. (In Russian). |
| [31] | Gomes A.P., Costa B., Marques R., Nunes V., Coelho C. Kaplan-Meier survival analysis: Practical insights for clinicians // Acta Med. Port. – 2024. – V.37(4). – P.280-285. |
| [32] | Jaeschke H., Bajt M. Intracellular signaling mechanisms of acetaminophen-induced liver cell death // Toxicol. Sci. – 2006. – V.89. – P.31-41. |
| [33] | Kaplan E.L., Meier P. Nonparametric estimation from incomplete observations // Journal of the American Statistical Association. – 1958. – V.53(282). – P.457-481. |
| [34] | Ke B.-J., . Lee C.-L. Cordyceps cicadae NTTU 868 mycelium prevents CCl4-induced hepatic fibrosis in BALB/c mice via inhibiting the expression of pro-inflammatory and pro-fibrotic cytokines // Journal of Functional Foods. – 2018. – V.43. – P.214-223. |
| [35] | Khadrawy S.M., Mohamed H.M., Mahmoud A.M. Mesenchymal stem cells ameliorate oxidative stress, inflammation, and hepatic fibrosis via Nrf2/HO-1 signaling pathway in rats // Environmental Science and Pollution Research. – 2021. – V.28. – P.2019-2030. |
| [36] | Kleinbaum D.G., Klein M. Survival analysis. A self‐learning text (3 Ed.) // In “Statistics for biology and health” (Ed. Gail M., Krickeberg K., Samet J.M., Tsiatis A., Wong W.). – “Springer” (New York, Dordrecht, Heidelberg, London). – 2020. – P.1-712. |
| [37] | Krylov D.P., Rodimova S.A., Karabut M.M., Kuznetsova D.S. Experimental Models for Studying the Structural and Functional State of the Liver during Pathology Development (Review) // Modern Technologies in Medicine. – 2023. – #15(4) – P.65-84. (In Russian). |
| [38] | Lakin G.F. Biometrics // Moscow. – “Higher School Publishing House”, 1990. – P.284. (In Russian). |
| [39] | Lebedeva E.I. Dynamics of Structural and Functional Disorders in the Liver of Rats with Experimental Cirrhosis // Bulletin of VSMU. – 2015. – #.14(3). – P.21-31. |
| [40] | Li J., Liu W., Zhang J., Sun C. The Role of mitochondrial quality control in liver diseases: Dawn of a therapeutic era // Int. J. Biol. Sci. – 2025. – V.10:21(4). – P.1767-1783. |
| [41] | Li X., Chen W., Jia Z., Xiao Y., Shi A., Ma X. Mitochondrial dysfunction as a pathogenesis and therapeutic strategy for metabolic dysfunction-associated steatotic liver disease // Int. J. Mol. Sci. – 2025. – V.26(4256). – P.1-29. |
| [42] | Lin S.-Y., Xu D., Du X.-X., Ran C.-L., Xu L., Ren S.-J., Tang Z.-T., Yin L.-Z., He C.-L., Yuan Z.-X., Fu H.-L., Zhao X.-L., Shu G. Protective effects of salidroside against carbon tetrachloride (CCl4)-induced liver injury by initiating mitochondria to resist oxidative stress in mice // Int. J. Mol. Sci. – 2019. – V.20(3187). – P.1-14. |
| [43] | Liu B., Fang Y., Yi R., Zhao X. Preventive effect of Blueberry extract on liver injury induced by carbon tetrachloride in mice // Foods. – 2019. – V.8(48). – Р.1-13. |
| [44] | Ma Y., Bao Y., Wu L., Ke Y., Tan L., Ren H., Song J., Zhang Q., Jin Y. IL-8 exacerbates CCl4-induced liver fibrosis in human IL-8-expressing mice via the PI3K/Akt/HIF-1α pathway // Molecular Immunology. – 2022. – V.152. – P.111-122. |
| [45] | Makhmudov L.U., Aripov T.F., Vypova N.L., Nurbekova N.B., Yuldashev H.A., Esanov R.S., Gafurov M.B., Yakubova R.A., Tagaygalieva N.A. Antioxidant and hepatoprotective properties of complexes of monoammonium salt of glycyrrhizic acid with amino acids and phenolic compounds // Experimental and clinical pharmacology. – 2023. – #.86(3). – P.23-28. (In Russian). |
| [46] | Mallaeva M.M., Mustafakulov M.A. Hepatitis is a powerful antioxidant enzyme that produces polyphenols // Computer Science and Engineering Technologies (Conference on Materials). – 2023. – #1(2). – 391-394. (In Uzbek). |
| [47] | Masuda A., Nakamura T., Abe M., Iwamoto H., Sakaue T., Tanaka T., Suzuki H., Koga H., Torimura T. Promotion of liver regeneration and anti‑fibrotic effects of the TGF‑β receptor kinase inhibitor galunisertib in CCl4‑treated mice // International Journal of Molecular Medicine. – 2020. – V.46. – P.427-438. |
| [48] | Miroshnikov M.V., Makarova M.N. Variability of blood biochemical parameters and the establishment of reference intervals in preclinical studies (Communication 4: Mice) // Laboratory animals for scientific research. – 2021. – #03. – P.63-69. (In Russian). |
| [49] | Mittra I., Pal K., Pancholi N., Tidke P., Siddiqui S., Rane B., D’souza J., Shaikh A., Parab S., Shinde S., Jadhav V., Shende S., Raghuram G.V. Cell-free chromatin particles released from dying host cells are global instigators of endotoxin sepsis in mice // PLoS ONE. – 2020. – V.15(3:e0229017). – P.1-22. |
| [50] | Mondal M., Sarkar C., Saha S., Hossain N., Norouzi R., Mubarak M.S., Siyadatpanah A., Wilairatana P., Hossain R., Islam M.T., Coutinho H.D.M. Hepatoprotective activity of Andrographolide possibly through antioxidative defense mechanism in Sprague-Dawley rats // Toxicology Reports. – 2022. – V.9. – P.1013-1022. |
| [51] | Nhung T.H., Nam N.H., Nguyen1 N.T.K., Huy L.M., Giang T.H., Nghia H., Van Thanh N. Establishment of a standardized mouse model of hepatic fibrosis for biomedical research // Biomedical Research and Therapy. – 2014. – V.1(2). – P.43-49. |
| [52] | Nishitani M., Okada H., Nio K., Hayashi T., Terashima T., Iida N., Shimakami T., Takatori H., Honda M., Kaneko S. et al. Mint3 as a molecular target activated in the early stage of hepatocarcinogenesis // Int. J. Mol. Sci. 2025. – V.26(1430). – P.1-15. |
| [53] | Nizinski P., Krajewska A., Oniszczuk T., Polak B., Oniszczuk A. Hepatoprotective effect of kaempferol – A review // Molecules. – 2025. – V.30(1913). – P.1-25. |
| [54] | Plokhinsky N.A. Biometrics // Moscow. – “Moscow State University Publishing House”. – 1970. – P.20-367. (In Russian). |
| [55] | Rafiq H., Ayaz M., Khan H.A., Iqbal M., Quraish S., Afridi S.G., Khan A., Khan B., Sher A., Siraj F., Shams S. Therapeutic potential of stem cell and melatonin on the reduction of CCl4-induced liver fibrosis in experimental mice model // Brazilian Journal of Biology. – 2024. – V.84(e253061). – P.1-7-б. |
| [56] | Rebrova O.Yu. Statistical Analysis of Medical Data (Application of the STATISTIKA Software Package). – Moscow. – “Media Sphere Publishing House”, 2002. – P.5-312. (In Russian). |
| [57] | Renner H. The limited relevance of models used for testing human hepatic diseases and their prevention (In: “Mechanisms of hepatocyte injury and death” Keppler E. et al. (Eds.) // MTP Press Ltd., Lancaster, 1985. – P.311-320. |
| [58] | Reynolds E.S. Liver parenchymal cell injury. I. Initial alterations of the cell following poisoning with carbon tetrachloride // J. Cell Biol. – 1963. – V.19. – P.139-157. |
| [59] | Richards S.J., Macdonald A.S. On contemporary mortality models (For actuarial use) // Ainslie Place, Edinburgh, 2024. – Р.80. |
| [60] | Ruz-Maldonado I., Gonzalez J.T., Zhang H., Sun J., Bort A., Kabir I., Kibbey R.G., Suarez Y., Greif D.M., C.Fernandez-Hernando. Heterogeneity of hepatocyte dynamics restores liver architecture after chemical, physical or viral damage // Nature Communications. – 2024. – V.15(1247). – P.1-24. |
| [61] | Salim N.S., Abo El-Maati M.F., Abdelnour S.A., Abdel-Alim M.E. Hepatoprotective activity of Taraxacum officinale extract against CCl4-induced liver injury in rats // Food Bioscience. – 2025. – V.68(106708). – P.1-12. |
| [62] | Scholten D., Trebicka J., Liedtke C., Weiskirchen R. The carbon tetrachloride model in mice // Laboratory Animals. – 2015. – V.49(S1). – P.4-11. |
| [63] | Sharashova E.E., Kholmatova K.K., Gorbatova M.A., Grzhibovsky A.M. Application of survival analysis in healthcare using the SPSS statistical software package // Science and Healthcare. – 2017. – #5. – P.5-28. (In Russian). |
| [64] | Shim J.Y., Kim M.H., Kim H.D., Ahn J.Y., Yun Y.S., Song J.Y. Protective action of the immunomodulator ginsan against carbon tetrachloride-induced liver injury via control of oxidative stress and the inflammatoryresponse // Toxicology and Applied Pharmacology. – 2010. – V.242(3). – P.318-325. |
| [65] | Shiryaeva A.P., Baidyuk E.V., Arkadieva A.V., Okovitiy S.V., Morozov V.I., Sakut G.A. State of liver mitochondrial respiratory chain in rats with experimental toxic hepatitis // Cell and Tissue Biology. – 2007. – V.1(2). – P.169-177. |
| [66] | Shorina E.D., Koshkina D.A., Grebnev D.Yu., Modeling of Liver Fibrosis in Mice // Proceedings of the VI International (76th All-Russian) Scientific and Practical Conference “Topical Issues of Modern Medical Science and Health Care”. – 2021. – P.1515-1520. |
| [67] | Singh D., Khan M.A., Siddique H.R. Unveiling the therapeutic promise of natural products in alleviating drug-induced liver injury: Present advancements and future prospects // Phytotherapy Research. – 2023. – V.39(1). – P.22-41. |
| [68] | Slater T.F. Activation of carbon tetrachloride: chemical principles and biological significance (In: “Free radicals, lipid peroxidation and cancer”, McBrien D.C.H., Slater T.F. (Eds.) // Academic Press, London, 1981. – Р.243-270. |
| [69] | Slinin A.S., Bydanov O.I., Karachunsky A.I. Analysis of survival and the probability of occurrence of individual events in patients with acute leukemia // Issues of hematology/oncology and immunopathology in pediatrics. – 2016. – #15(3). – P.34-39. (In Russian). |
| [70] | Soguyko Yu.R., Krivko Yu.Ya., Krikun E.N. Ultrastructural features of the liver of rats in norm and in experimental diabetes mellitus at late stages of the course // Scientific news (Series Medicine. Pharmacy). – 2013. – #4(147). – Issue. 21. – P.147-150. (In Russian). |
| [71] | Tabet E., Genet V., Tiaho F., Lucas-Clerc C., Gelu-Simeon M., Piquet-Pellorce C., Samson M. Chlordecone potentiates hepatic fibrosis in chronic liver injury induced by carbon tetrachloride in mice // Toxicol. Lett. – 2016. – V.25(255). – P.1-10. |
| [72] | Timonin A.N., Nikitin N.S., Apryatin S.A., Trebukh M.D. Analysis of survival of ICR-1 mice in a biomodeling of starvation using carbon tetrachloride in an in vivo experiment // Issues of nutrition. – 2018. – #.87(5). – P.267-268. (In Russian). |
| [73] | Titova A.A., Bilyalov A.I., Kiyasov A.P., Titova M.A. Laboratory animals for scientific research // Kazan (Kazan University), 2021. – P.71. (In Russian). |
| [74] | Udut V.V., Vengerovsky A.I., Korshunov D.A., Karkishchenko N.N. The Effect of Phospholipid Hepatoprotectors on Bioenergetics and Lipid Peroxidation in the Liver in Experimental Pathology Induced by Paracetamol // Biomedicine. – 2012. – №1. – P.120-127. |
| [75] | Wang B., Cui S., Mao B., Zhang Q., Tian F., Zhao J., Tang X., Chen W. Cyanidin Alleviated CCl4-induced acute liver injury by regulating the Nrf2 and NF-κB signaling pathways // Antioxidants. – 2022. – V.11(2383). – P.1-14. |
| [76] | Xue Y., Deng Q., Zhang Q., Ma Z., Chen B., Yu X., Peng H., Yao S., Liu J., Ye Y., Pan G. Gigantol ameliorates CCl4‑induced liver injury via preventing activation of JNK/cPLA2/12‑LOX inflammatory pathway // Scientific Reports. – 2020. – V.10(22265). – P.1-13. |
| [77] | Yakovenko E.P., Yakovenko A.V., Ivanov A.N. et al. Drug-Induced Liver Damage. Diagnostics and Treatment // Attending Physician. – 2011. – #2. – P.16-20. (In Russian). |
| [78] | Yu C., Wang F., Jin C., Wu X., Chan W.-K., McKeehan W.L. Increased carbon tetrachloride-induced liver injury and fibrosis in FGFR4-deficient mice // American Journal of Pathology. – 2002. – V.161(6). – Р.1-8. |
| [79] | Zapadnyuk I.P., Zapadnyuk V.I., Zakharia E.A., Zapadnyuk B.V. Laboratory Animals. Breeding, maintenance, experimental use (3rd edition, revised and enlarged) // Kyiv. – Publishing house “Vishcha shkola”, 1983. – 380 p. (In Russian). |
| [80] | Zhang L., Liu C., Yin L., Huang C., Fan S. Mangiferin relieves CCl4‑induced liver fibrosis in mice // Scientific Reports. – 2023. – V.13(4172). – P.1-9. |
| [81] | Zhurakulov Sh.N. Tetrahydroisoquinoline, quinazolone alkaloid, its synthesizing and modification in biology // Thesis of dissertations by doctors (DSc). – Tashkent, 2023. – P.5-74. (In Uzbek). |
| [82] | Zhurakulov Sh.N., Babkin V.A., Chernyak E.I., Morozov S.V., Grigorev I.A., Levkovich M.G., Vinogradova V.I. Aminomethylation of 1-aryl-6,7-dimetoxy-1,2,3,4-tetrahydroisoquinolines by dihydroquercetin // Chemistry of Natural Compounds. – 2015. – V.51(1). – P.57-61. |
| [83] | Zhurakulov Sh.N., Vinogradova V.I., Levkovich M.G. Synthesis of 1-aryltetrahydroisoquinoline alkaloids and their analogs // Chemistry of Natural Compounds. – 2013. – V.49(1). – P.70-74. |


 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML