Sobirova Gulrukh Khasan qizi1, Mukhtorjonova Fotimajon Muzaffar qizi2, Sulaymanov Sherali Abdupattayevich3, Komilov Bakhrom Jamoldinovich4, Pozilov Mamurjon Komiljonovich2
1Fergana State University, Department of Zoology and main biology, Fergana, Uzbekistan
2National University of Uzbekistan named after Mirzo Ulugbek, Department of Biophysics, Tashkent, Uzbekistan
3Namangan State University, Department of Chemistry, Namangan, Uzbekistan
4Namangan State Pedagogical Institute, Department of Natural Sciences, Namangan, Uzbekistan
Correspondence to: Sobirova Gulrukh Khasan qizi, Fergana State University, Department of Zoology and main biology, Fergana, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
In vivo experiments were conducted to study the effect of flavonoids chrysoeriol and rhamnocitrin on the level of glucose and insulin in rat blood plasma, as well as on the activity of mitochondrial antioxidant liver enzymes - superoxide dismutase (SOD) and catalase under conditions of streptozotocin (STZ) diabetes. Mitochondria from outbred albino rats were isolated by differential centrifugation. Flavonoids chrysoeriol and rhamnocitrin, as well as the flavonoid quercetin with antidiabetic effect were administered orally at a dose of 30 mg/kg for 10 days to rats with STZ- induced diabetes. Experiments have shown that the flavonoids chrysoeriol and rhamnocitrin reduce plasma glucose levels and increase insulin levels in STZ-induced diabetes. They have also been shown to effectively influence the activity of SOD and catalase enzymes in the liver mitochondria of rats with STZ-induced diabetes and possess antioxidant properties.
Keywords:
Liver, Mitochondria, Glucose, Insulin, SOD, Catalase, Quercetin, Chrysoeriol, Rhamnocitrin
Cite this paper: Sobirova Gulrukh Khasan qizi, Mukhtorjonova Fotimajon Muzaffar qizi, Sulaymanov Sherali Abdupattayevich, Komilov Bakhrom Jamoldinovich, Pozilov Mamurjon Komiljonovich, Antioxidant and Antidiabetic Effect of Flavonoids Chrysoeriol and Rhamnocitrin, International Journal of Virology and Molecular Biology, Vol. 14 No. 6, 2025, pp. 146-150. doi: 10.5923/j.ijvmb.20251406.11.
1. Introduction
Diabetes mellitus is a chronic, non-communicable disease caused by impaired glucose and lipid metabolism. According to the latest data from the International Diabetes Federation (IDF), 537 million people aged 20 to 79 suffer from diabetes [1; 1-16 p.]. Flavonoids are biologically active compounds known as secondary plant metabolites, low-molecular-weight phenolic compounds. They are widely distributed in the roots, stems, leaves, flowers, and fruits of plants [2; 28 p.]. They have a variety of biological activities, including organoprotective, hypoglycemic, hypolipidemic, antioxidant and anti-inflammatory effects.The antidiabetic properties of flavonoids regulate carbohydrate absorption, insulin signaling, insulin secretion, glucose uptake, and fat accumulation [3; 60 p.]. They affect numerous molecules and control the activity of several signaling pathways, such as enhancing β-cell proliferation, stimulating insulin secretion, reducing apoptosis, and decreasing hyperglycemia by regulating glucose metabolism in the liver. They also act as antioxidants, modulating oxidative stress in the body by neutralizing the effects of nitrogen and oxygen radicals, thereby preventing the development of diseases [4; 569 p.].Mitochondria are the primary source of reactive oxygen species (ROS), and under conditions of chronically high glucose levels, disruption of the electron transport chain in the inner mitochondrial membrane leads to the formation of free radicals. This increases proton flux and alters the potential of mitochondrial membrane, which in turn leads to the release of cytochrome c (Cyt-c), leading to apoptosis. [5; 1-10 p.]. These pathophysiological processes lead to increased lipid peroxidation (LPO) in mitochondrial membranes. Cells are protected from LPO by a system of antioxidant enzymes. The most important protective enzymes are SOD, catalase, glutathione reductase, and glutathione peroxidase. They neutralize the main and intermediate products of lipid peroxidation, as well as secondary products of lipid peroxidation of glutathione transferase, glyoxidase, formaldehyde dehydrogenase and other carbonyl compounds [6; 2786-2791 p.]. Currently, the study of the mechanisms of action of biologically active compounds that neutralize free radicals generated by the mitochondrial respiratory chain and membrane disturbances in cells and organelles in diabetes mellitus is one of the most promising areas of physiology and pharmacology. This article examines the effects of flavonoids chrysoeriol and rhamnocitrin on blood glucose and insulin levels, as well as the activity of antioxidant enzymes (SOD, catalase) in liver mitochondria in the STZ-diabetes model.
2. Materials and Methods
Flavonoids with hypoglycemic properties were selected through screening. Rhamnocitrin was isolated from the plant Oxytropis rosea, and the flavonoid chrysoeriol was isolated from the plant Centaurea squarrosa [Fig. 1]. The existing hypoglycemic compound quercetin was used as a standard prototype in the experiment. 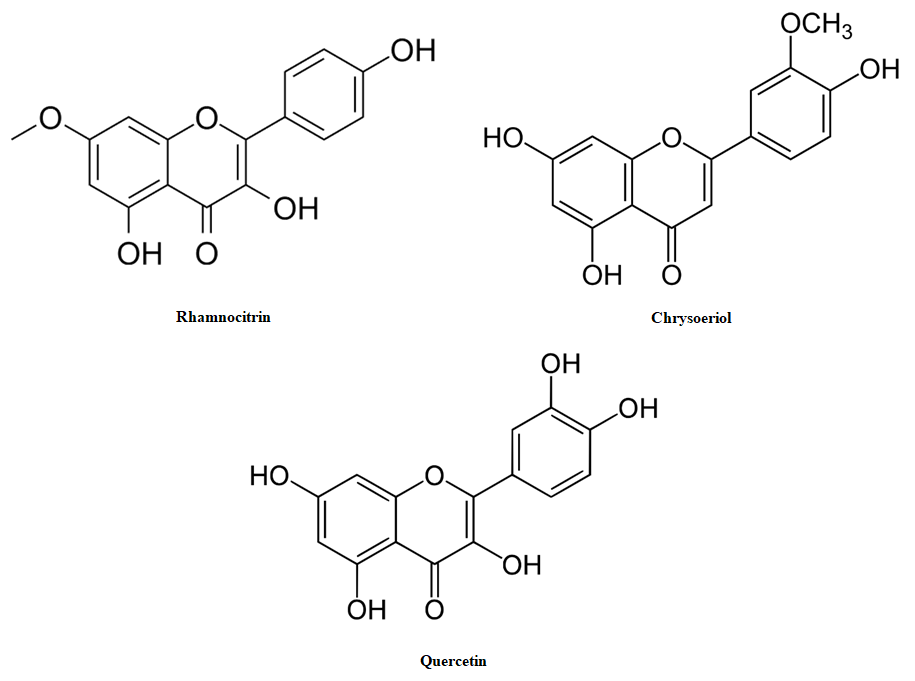 | Figure 1. Structures of the flavonoids used in this study |
The experiments were conducted on male outbred albino rats weighing 180-200 g. Scientific research on experimental animals was carried out in accordance with the International Helsinki Declaration, the rules developed by the Council for International Organizations of Medical Sciences (CIMO) (1985), and the “Bioethical Rules for the Use of Laboratory Animals in Scientific Research” (2019) of the Institute of Biophysics and Biochemistry. The experimental animals were divided into groups: group I – control (healthy), group II – experimental (STZ-diabetes), group III – experimental (STZ-diabetes + rhamnocitrin), group IV – experimental (STZ-diabetes + chrysoeriol) and group V (STZ-diabetes + quercetin). To induce diabetes in rats of groups II, III, IV and V after a day of fasting, a solution of STZ was administered subcutaneously into the abdominal cavity at a dose of 50 mg/kg (0.1 mol/L citrate buffer, 0.2 mL, pH 4.5) once [7; 18-24 p., 8; 160-170 p.]. Blood samples were taken from animals with STZ-induced diabetes every 3 days to determine glucose levels. After blood glucose levels exceeded 11 μmol/L following STZ injection (12 days), animals in group II were administered 0.2 mL of a 0.9% NaCl solution once daily. Group III was given rhamnocitrin at a dose of 20 mg/kg, group IV was given chrysoeriol flavonoids at a dose of 20 mg/kg, and group V was given quercetin flavonoids at a dose of 30 mg/kg once daily for 10 days. In animals with STZ diabetes mellitus, during pharmacotherapy (reducing blood glucose levels to 11 μmol/L), SOD and liver mitochondrial catalase activity were determined. Blood glucose levels were determined using the glucose oxidase method.Insulin levels in rat plasma were determined using an ELISA reagent kit with a standard insulin concentration range of approximately 0.01 ng/ml. The dynamic range for this assay is 0.156–10 ng/mL.Determination of the activity of the SOD enzyme (EC 1.15.1.1) was carried out using the method of Misra and Ya. Fridovich (1972) [9; 16-19 p.]. The method is based on the reduction of nitro tetrazole blue in an alkaline medium. Enzyme activity is expressed as units/min/mg protein.Catalase protects the body from the toxic effects of hydrogen peroxide, which is formed as a result of biological oxidation in its tissues. In liver mitochondria, the enzyme catalase exhibits very high catalytic activity. Color intensity was measured spectrophotometrically at 410 nm relative to a sample containing 2 ml of H₂O instead of H₂O₂. Catalase activity in liver mitochondria is expressed as μCat/mg protein [10; 16-18 p.].Statistical analysis of the results was performed using Origin 6.1 software (USA). Results were calculated as the arithmetic mean of five replicates. Differences between values obtained in in vivo experiments were calculated using Student's t-test. Values of *P<0.05 and **P<0.01 indicate statistical significance.
3. Results and Their Analysis
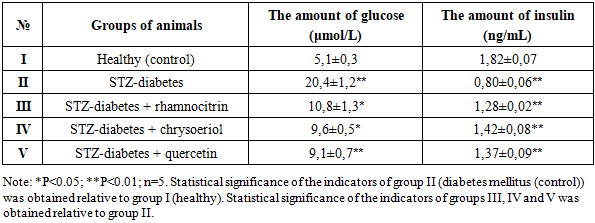
In type 2 diabetes, elevated plasma glucose levels are caused by impaired insulin secretion by pancreatic β-cells. Impaired insulin secretion may be due to impaired ion transport in the pancreatic plasma membrane or decreased ATP synthesis in the mitochondria. Blood was collected from the tails of experimental rats, and glucose concentrations were measured every 3 daysAfter blood glucose levels were reduced to levels close to control levels, the rats were decapitated. During the experiments, plasma glucose levels in healthy control rats (Group I) were 5.1 μmol/L. However, in Group II rats, which developed STZ-induced diabetes, plasma glucose levels were found to be 20.4 μmol/L, four times higher than the control value.Rats of group III with STZ-diabetes were orally administered rhamnocitrin, and rats of group IV were given the flavonoid chrysoeriol at a dose of 20 mg/kg for 10 days; the experiments were conducted after the glucose level had decreased. It was found that as a result of the administration of the flavonoid rhamnocitrin to rats of group III with STZ-diabetes, the blood glucose level in rats decreased to 10.8 mmol/l, and in rats of group IV – chrysoeriol – to 9.6 mmol/l. It was found that in rats of group V with STZ diabetes, which were administered the existing hypoglycemic flavonoid quercetin, the blood glucose level decreased to 9.1 mmol/l (Table 1).Table 1. Comparative assessment of the effect of flavonoids rhamnocitrin and chrysoeriol with quercetin on the level of glucose and insulin in the blood plasma of rats with type 2 diabetes mellitus (M±m)
 |
| |
|
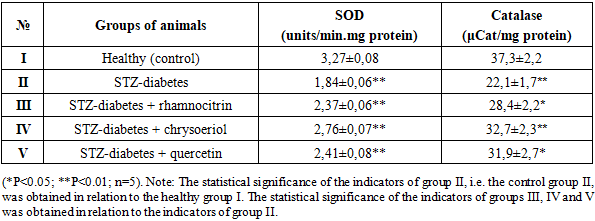
Glucose is the body's most important energy source. Insulin, produced by the islet cells of the pancreas, promotes glucose uptake into tissue cells. Insulin deficiency or decreased insulin activity leads to elevated blood glucose levels. Elevated plasma glucose concentrations are observed in diabetes mellitus (insulin-dependent and non-insulin-dependent) and other diseases and syndromes. Elevated blood glucose levels in rats with type 2 diabetes indicate decreased secretion of the hormone insulin by the pancreas. The hypoglycemic properties of the flavonoids rhamnocitrin and chrysoeriol in STZ-induced diabetes can be assessed by increasing insulin secretion. To confirm this hypothesis, our next experiment determined the effect of flavonoid compounds on blood insulin levels in STZ-induced diabetes. According to the results obtained, the plasma insulin level of healthy rats (group I), used as a control, was 1.82 ng/ml. However, the plasma insulin level of group II rats, in which diabetes was induced, was 0.80 ng/ml, which is 56.0% lower than the control value.Rats of group III with STZ-induced diabetes were orally administered rhamnocitrin, while rats of group IV were administered the flavonoid chrysoeriol. After a decrease in glucose levels, insulin levels were measured. It was found that administration of the flavonoid rhamnocitrin to rats of group III with STZ-induced diabetes increased the blood insulin level to 1.28 ng/ml, while in rats of group IV with chrysoeriol, it increased to 1.42 ng/ml. In rats of group V with STZ-induced diabetes, which were administered the existing hypoglycemic flavonoid quercetin, the blood insulin level increased to 1.37 ng/ml (Table 1). It was found that the effect of the flavonoid chrysoeriol on reducing glucose levels and increasing insulin levels under conditions of STZ diabetes was more active than rhamnocitrin, and almost the same as that of quercetin. In this case, one of the mechanisms by which flavonoid compounds reduce blood glucose concentrations and increase insulin levels in STZ-diabetic rats after pharmacotherapy may be the restoration of carbohydrate metabolism between the blood and liver by altering glycogenesis processes.Chronically elevated blood glucose levels cause oxidative stress on cells and tissues [11; 1-8 p.]. However, tissues, including hepatocytes, contain powerful antioxidant enzymes such as SOD, catalase, glutathione-S-transferase, and glutathione peroxidase, which not only neutralize free radicals but also protect liver cells from oxidative damage. Studies have shown that decreased SOD and catalase activity during hyperglycemia leads to increased levels of ROS, which ultimately leads to oxidative damage to the liver [12; 15-27 p.].Antioxidant defense mechanisms that eliminate reactive oxygen species (ROS) are essential for maintaining redox balance. Among the antioxidant enzymes actively involved in this process, the activity of SOD, glutathione peroxidase, catalase, and peroxiredoxin may be altered in STZ-induced diabetes. To address this issue, our next experiment examined the effects of the flavonoids rhamnocitrin and chrysoeriol on the activity of the antioxidant enzymes SOD and catalase in liver mitochondria in STZ-induced diabetes. Currently, studying the mechanisms of action of biologically active compounds that neutralize free radicals produced by the mitochondrial respiratory chain and membrane damage in cells and organelles in diabetes mellitus is one of the most promising areas of physiology and pharmacology. Flavonoid compounds have been shown to indiscriminately inhibit lipid peroxidation in liver mitochondria or reduce MDA formation in STZ-induced diabetes. However, the experiments conducted do not fully explain the effect of rhamnocitrin and chrysoeriol on antioxidant enzyme activity in STZ-induced diabetes. This requires further experimentation. To this end, our subsequent experiments examined the effect of the flavonoids chrysoeriol and rhamnocitrin on the activity of antioxidant enzymes (SOD, catalase) in liver mitochondria in a model of STZ-induced diabetes.First, the activity of the enzyme SOD was determined. SOD enzymes are known to play a key role in maintaining cellular homeostasis by catalyzing the conversion of superoxide radicals to molecular oxygen and hydrogen peroxide. The results revealed the influence of biological compounds on SOD enzyme activity in rat liver mitochondria under STZ-induced diabetes. The SOD level in the liver mitochondria of healthy rats which is given as a group I, used as a control, was 3.27±0.08 units/min.mg protein. It was found that the amount of SOD in the liver mitochondria of rats of group II, in which STZ-induced diabetes was 1.84±0.06 units/min.mg protein, which is 43.7% lower than in the control group I. With oral administration of the flavonoid rhamnocitrin to rats of group III with STZ-induced diabetes at a dose of 20 mg/kg for 10 days, the SOD content in the liver mitochondria of rats was 2.37±0.06 units/min.mg protein, which is 28.8% higher than in group II, i.e. in the control group (Table 2).Table 2. Comparative effect of flavonoids rhamnocitrin and chrysoeriol with quercetin on the activity of antioxidant enzymes - SOD and catalase - in the liver mitochondria of rats with STZ-diabetes
 |
| |
|
Group IV rats with STZ-induced diabetes were orally administered chrysoeriol at a dose of 20 mg/kg for 10 days. After this, the rats were decapitated, mitochondria were isolated from the liver, and SOD activity was determined. The SOD content in the liver mitochondria of rats with STZ-induced diabetes was 2.76±0.07 units/min.mg protein, which was 50.0% higher than the values in Group II, i.e., with STZ-induced diabetes (Table 2). It was found that quercetin administration to rats of group V with STZ-induced diabetes resulted in a 28.8% increase in SOD activity in the liver mitochondria compared to Group II. Thus, in STZ-induced diabetes, SOD activity in the liver mitochondria of rats decreases. The flavonoids rhamnocitrin and chrysoeriol increased SOD activity in the liver mitochondria in STZ-induced diabetes.The effect of flavonoid compounds on the activity of the catalase enzyme in the liver mitochondria of rats with STZ-induced diabetes was studied. According to the data obtained, the catalase content in the liver mitochondria of rats with STZ-induced diabetes in group II was 22.1±1.7 μCat/mg protein, and its activity increased by 40.7% compared to the control group (group I) (Table 2). When rhamnocitrin was administered to rats of group III with STZ diabetes, the amount of catalase in the mitochondria of the rat liver was 28.4±2.2 μCat/mg protein, which is 28.5% more than in group II, i.e. with STZ diabetes. After oral administration of chrysoeriol at a dose of 20 mg/kg to rats of group IV with STZ-diabetes for 10 days, the rats were decapitated, mitochondria were isolated from the liver and catalase activity was determined. Moreover, the amount of catalase in the liver mitochondria of rats with STZ-diabetes under the influence of the chrysoeriol compound was 32.7±2.3 μCat/mg protein, which is 47.9% higher than in STZ-diabetes (group II). It was found that the administration of quercetin (31.9±2.7 μCat/mg protein) to rats of group V with STZ-diabetes increased the activity of the catalase enzyme in the liver mitochondria by 44.3% compared to the values in group II, i.e. in the control (Table 2).In diabetes, there is a complex relationship between SOD and catalase enzymes, as they play a critical role in combating oxidative stress caused by hyperglycemia. Some studies show that while the body exhibits high enzyme activity in response to stress, others show that long-term stress can lead to decreased SOD and catalase activity. Decreased activity of antioxidant enzymes such as SOD and catalase leads to tissue damage and mitochondrial damage.The isolated flavonoids rhamnocitrin and chrysoeriol led to some restoration of the activity of enzymes of liver mitochondria SOD and catalase in STZ diabetes. Scientists suggest that activating the cellular antioxidant system with bioactive compounds may become an important strategy in the treatment of diabetes and its complications. Pharmacological correction of membrane disorders of liver mitochondria in diabetes mellitus and other pathological conditions using biologically active compounds isolated from plants is becoming one of the promising areas of physiology and medicine.
4. Conclusions
Flavonoids rhamnocitrin and chrysoeriol have shown hypoglycemic properties, reducing blood glucose levels, increasing insulin levels and antioxidant enzyme activity in STZ-induced diabetes. It has been statistically established that the hypoglycemic activity of rhamnocitrin in STZ diabetes is close to the activity of quercetin, but the hypoglycemic, insulin-increasing, strong antioxidant and antidiabetic effects of chrysoeriol are somewhat more pronounced.It has been statistically established that the hypoglycemic activity of rhamnocitrin in conditions of STZ diabetes is close to that of quercetin, but the hypoglycemic, insulin-increasing, strong antioxidant and antidiabetic effects of chrysoeriol are more pronounced.
References
| [1] | Yi X., Dong M., Guo N., Tian J., Lei P., Wang S., Yang Y., Shi Y. Flavonoids improve type 2 diabetes mellitus and its complications: a review // Front. Nutr. – 2023 – V. 31 – P. 1-16. |
| [2] | Abotaleb M., Samuel S.M., Varghese E., Varghese S., Kubatka P., Liskova A., Busselberg D. Flavonoids in Cancer and Apoptosis. // Cancers (Basel) – 2018 – V. 11: – P. 28. |
| [3] | Vinayagam R., Xu B. Antidiabetic properties of dietary flavonoids: A cellular mechanism review // Nutr. Metab. (Lond.) – 2015 – V. 12: – P. 60. |
| [4] | Kawser Hossain M., Abdal Dayem A., Han J., Yin Y., Kim K., Kumar Saha S., Cho S.G. Molecular Mechanisms of the Anti-Obesity and Anti-Diabetic Properties of Flavonoids // Int. J. Mol. Sci. – 2016 – V. 17: – P. 569. |
| [5] | Zhang Z., Huang Q., Zhao D., Lian F., Li X., Qi W. The impact of oxidative stress-induced mitochondrial dysfunction on diabetic microvascular complications // Front Endocrinol (Lausanne) – 2023 – V. 14: – P. 1-10. |
| [6] | Kaneko H. Pyrethroids: Mammalian metabolism and toxicity // Journal of Agricultural and Food Chemistry. – 2011. – V. 59(7): – P. 2786-2791. |
| [7] | Palchikova N.A., Kuznetsova N.V., Kuzminova O.I., Kelyatitskaya V.G. Hormonal and biochemical characteristics of alloxan- and streptozotocin-induced models of experimental diabetes // Bulletin of the Siberian Branch of the Russian Academy of Medical Sciences. – 2013. – Vol. 33. – No. 6. – P. 18–24. |
| [8] | Indumathi D., Sujithra K., Srinivasan S., Vinothkumar V. Protective effect of betanin against streptozotocin-Nicotinamide induced liver, kidney and pancreas damage by attenuating lipid byproducts and improving renal biomarkers in wistar rats // Int. J. Adv. Res. Biol. Sci – 2017: – V. 4: – P. 160–170. |
| [9] | Matyushin B.N. Determination of superoxide dismutase activity in puncture biopsy material of the liver in chronic liver damage // Laboratory Practice. – 1991. – No. 7. – P. 16–19. |
| [10] | Korolyuk M.A., Ivanova L.I., Maiorova I.G., Tokarev V.E. Methods for the determination of catalase activity. – Moscow: Meditsina, 1988. – P. 16–18. |
| [11] | Kumar K.J., Nikitha P., Bendi M., Karnik M., Madhunapantula S.V. Comparative evaluation of oxidative stress biomarkers and antioxidant enzymes in children with or without type 1 diabetes // Results in Chemistry – 2025 – V. 17 – P. 1-8. |
| [12] | Xingyu C., Na X., Lixiang F., Yujing H., Yuyao W., Huili Z., Jing T., Yuanyuan Z. Oxidative stress in diabetes mellitus and its complications: From pathophysiology to therapeutic strategies // Chinese Medical Journal – 2025 - V. 138 (1): – P. 15-27. |



 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML
