-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Virology and Molecular Biology
p-ISSN: 2163-2219 e-ISSN: 2163-2227
2025; 14(6): 123-127
doi:10.5923/j.ijvmb.20251406.07
Received: Sep. 26, 2025; Accepted: Oct. 22, 2025; Published: Oct. 31, 2025

Morphofunctional Characteristics of the Tumor Microenvironment in Breast Cancer Precursors
Khomidova Tursunoy Ergashboy qizi1, Karimov Azizbek Ravshanbekovich2, Isroiljonov Saminjon1
1Fergana State University, Fergana, Uzbekistan
2Fergana Regional Branch of the Republican Specialized Scientific and Practical Medical Center of Oncology and Radiology, Fergana, Uzbekistan
Correspondence to: Khomidova Tursunoy Ergashboy qizi, Fergana State University, Fergana, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Currently, breast cancer is one of the most common oncological diseases worldwide. It ranks high in terms of women's health and mortality rates. This disease has a serious impact on women's health and lifestyle. One of the main ways to prevent breast cancer is early diagnosis. The earlier the disease is detected, the more effective the treatment is up to 90%. Analysis of precancerous diseases is of great importance in preventing the development of cancer. Objective. To analyze the characteristics of the tumor microenvironment in the development of cancer. Materials and methods. Family history of the disease, complaints, objective and physical examination methods, laboratory tests, analysis of breast tissue by immunohistochemical method of female patients aged 25-65 years. Results and discussion. The influence of tumor microenvironment components on hormone-dependent and inflammatory processes in breast tissue of female patients was analyzed, changes in biomarkers were detected, which indicate that chronic inflammation and stress-related mechanisms, along with hormonal imbalance, play a key role in the development of breast cancer. Conclusion. The tumor microenvironment plays a significant role in precancerous conditions of the breast and in the development of cancer. Analysis of the amount of microenvironment biomarkers plays a crucial role in the effective treatment of cancer.
Keywords: Breast cancer, Biomarker, ki-67, p53, Hyperplasia, Tumor microenvironment, Benign, Malignant, Fertile, Menopause
Cite this paper: Khomidova Tursunoy Ergashboy qizi, Karimov Azizbek Ravshanbekovich, Isroiljonov Saminjon, Morphofunctional Characteristics of the Tumor Microenvironment in Breast Cancer Precursors, International Journal of Virology and Molecular Biology, Vol. 14 No. 6, 2025, pp. 123-127. doi: 10.5923/j.ijvmb.20251406.07.
1. Introduction
- In recent years, breast cancer has become one of the leading causes of cancer death among women [1]. In precancerous conditions of the breast, benign hormonal and inflammatory diseases of the mammary gland are of great importance [9]. It has been shown that if breast cancer is detected in the early stages, the mortality rate is reduced to 17% in patients over 40 years of age and to 30% in patients over 50 years of age. Disability is reduced and the life expectancy of women is extended [10]. When analyzing diseases caused by hormonal changes in precancerous conditions of the breast, changes in the amount of hormones are detected. Recent studies have shown that the main cause of breast cancer development is not hormonal imbalance and genetic factors, but the tumor microenvironment (TME). In precancerous breast diseases - hormonal and inflammatory diseases, cellular changes in the microenvironment are a key factor in the development of cancer [5].In addition to cancer cells, tumors display another aspect of complexity: they contain a repertoire of seemingly normal cells that contribute to the acquisition of specific characteristics by creating a “tumor microenvironment”. The tumor microenvironment is a complex system consisting of stromal cells, fibroblasts, immune system cells, i.e. lymphocytes, and blood vessels in the tissues where cancer cells reside [3]. In assessing the morpho-functional characteristics of precancerous breast conditions, biomarkers such as proliferation index Ki-67, and tumor growth arresting protein p53 and endothelial biomarkers are important for the prevention and treatment of cancer development [6,2,3].Ki-67 is a marker of cell proliferation activity, and high levels of Ki-67 are associated with aggressiveness and rapid growth of the tumor [4].Similarly, high levels of Ki-67 in the tumor microenvironment lead to increased activity of stromal cells, fibroblasts, angiogenesis, and immune cells [8]. P53 acts as a “braking mechanism” in the tumor microenvironment, and mutation of p53 leads to loss of this control. Mutation of p53 leads to an increase in reactive oxygen species (ROS) in the tumor microenvironment and the production of factors that stimulate angiogenesis [7].
2. Research Methods
- The study was conducted in the laboratory of the Fergana regional branch of the Republican Specialized Scientific and Practical Medical Center of Oncology and Radiology. (N=236 patients) The material for immunohistochemical examination (IHC) is the tissue obtained as a result of sampling material from the patient (biopsy, surgical material, etc.), i.e. a paraffin section on a slide. The patient's tissue is subjected to standard histological processing and goes through all the stages of analysis: taking the material, cutting, fixing in 10% buffered formalin, wiring, pouring into paraffin, microtomy, mounting the slice on a slide. Studies on frozen sections (cryo-sections) and on cellular material are also possible. Specialists of the medical laboratory prepare samples and describe biological structures using immunohistochemical analyses. Based on the analysis, the attending physician receives the conclusion of histological analyses with positive and negative markers, and this helps him to confirm or refute the conclusions from other clinical and laboratory studies.Immunohistochemical staining (IHC) consists of several stages:1. Dewaxing and rehydration of the slice.Dewaxing is the process of hydration (saturation with water) of a tissue fragment and the replacement of paraffin with an aqueous component. Sample dewaxing is a standard routine procedure in a histology laboratory. The study includes the stages of treating the paraffin section with xylene and alcohol in order for the antibodies to penetrate into the tissue and bind to antigenic epitopes. Currently, it is possible to carry out de-refining using special buffers.2. Unmasking. During fixation, tissue antigens undergo conformational changes associated with protein coagulation and the formation of cross-links when exposed to aldehydes. These changes mask tissue antigens and make them inaccessible to antibodies. Unmasking is the process of restoring the antigenic activity of a tissue that has changed during sample fixation. The mechanism of unmasking is the destruction of methylene bridges (-CH2-) in proteins.Unmasking can be of 2 types:High-temperature, occurs using special buffers for unmasking with different pH (EDTA and Citrate buffers) Proteolytic, occurs using enzymes (proteases) of the equipment for the unmasking stage, a water bath or a pressure cooker are required, as well as containers with glass holders.3. Washing the slice. Flushing is necessary to remove reagent residues from the slice. There are and are used special buffers for washing, they can be phosphate-salt (PBS) or tris (TBS) in composition. They often contain Tween-20 in their composition, which contributes to more efficient washing of the slice, as a result of which the nonspecific background staining of the sample is reduced.4. Blocking. The IHC stage is necessary to block the endogenous peroxidase located in the tissue and eliminate the nonspecific binding of reagents to tissue components before applying the primary antibody. Blocking solutions (Peroxide Block) are used for this purpose. They usually consist of a 0.3% solution of hydrogen peroxide (H2O2) with the addition of sodium azide as a preservative.The mechanism of action of IHC is hydrogen peroxide binds endogenous peroxidase, and it does not act as a substrate for horseradish peroxidase at the stage of antigen visualization. The result of the analysis is the absence of nonspecific and background staining of the slice.5. Application of the primary antibody. Primary antibodies are the main reagent in the immunohistochemical study when setting up an immunohistochemical reaction. This is due to their ability to bind highly specifically to the antigen that caused their formation. The degree of binding depends on the properties and structure of the molecules. During the study, the antibody does not bind to the entire antigen, but to its specific site, the epitope. According to the population, it can be divided into heterogeneous and homogeneous, and according to its specificity to epitopes– into polyclonal antibodies and monoclonal antibodies.Polyclonal antibodies are a heterogeneous mixture of antibodies directed against different epitopes of the same antigen, which are formed by different clones of animal B cells and, as a result, have different immunochemical properties. Due to their multiclonality, they can recognize multiple epitopes on a single protein molecule during analysis, i.e. they are highly sensitive, but the specificity of polyclonal antibodies is lower.Monoclonal cells are a homogeneous population of immunoglobulins directed against a single epitope. which are formed by a single clone of B cells from the same animal and are therefore similar in immunochemical properties. They have high specificity, but their sensitivity is lower.Primary antibodies can be concentrated in format – they must be diluted in a special diluent for antibodies and ready for use (Ready-to-Use, RTU) - they do not require dilution and are immediately ready to be applied to a slice. Compared to concentrated, ready-to-use (RTU) antibodies have a shorter shelf life.Information about the pH of the unmasking buffer, the dilution range, the incubation time and temperature with primary antibodies is contained in the manufacturer's instructions.6. Antigen visualization. Antigen detection in the tissue can be carried out by direct method (immunofluorescence) or indirect method (enzymatic reaction).Primary antibodies labeled with fluorescent dyes are used in immunofluorescence. This method of IHC examination allows detecting surface antigens, as well as determining their exact localization. The results of the reaction are visualized using a fluorescence microscope. Chromogenic visualization of reaction results is a much more popular research method. In this case, the assessment is performed in the light field of a classical microscope and does not require additional equipment.The mechanism of chromogenic antigen visualization is a reaction involving an enzyme and its substrate, followed by the formation of a colored product under the influence of chromogen. This research method consists of several stages. After incubation with unconjugated primary antibodies, secondary antibodies labeled with an enzyme (detection system) are applied. Next, the slice is treated with a solution of substrate and chromogen so that the enzyme, substrate and chromogen react. As a result of the enzymatic reaction, a reaction product is obtained - a colored precipitate, which is deposited at the site of localization of the desired antigen in the tissue, giving the cut a color.7. Touch-up and conclusion. The final stage of immunohistochemical staining (IHC) is to tint the drug with hematoxylin for better orientation in the drug, as well as enclose the drug under a cover glass or film.Immunohistochemical examination of IHC is important for the diagnosis and determination of disease factors, including genetic and oncological tumor pathologies (cancer), and helps doctors analyze and predict the treatment of malignant tumors (breast cancer) and other oncological diseases.
3. Results and Discussion
- In the analysis of the tumor microenvironment in women with precancerous breast tumors of fertile and menopausal age, markers such as CD31, VEGFR, P53, Ki67 were used. The levels of biomarkers were compared with precancerous breast tumors and inflammatory diseases.The CD31 content in women with fertile age FCM was 4 nmol / L. The amount of CD31 in women with fertile age mastitis was 20.8 nmol / L. Women with fkm in fertile age were found to have 5.2 times less CD31 when taken compared to inflammation. In women with fibroadenoma of fertile age, the CD31 content was 2 nmol/L. In women with fertilised age mastitis, the CD31 content was found to be 20.8 nmol/l, and the CD31 content in fibroadenoma patients was found to be 10.4 times less than in inflammatory diseases. In menopausal aged fkm patients, the CD31 content in women was 10.3 nmol / L. The CD31 content was 29 nmol / L in menopausal aged mastitis patients (Figure 1). Women with menopause age FCM were found to have CD31 levels 2.8 times lower when taken compared to inflammation.n menopausal aged fkm patients, the CD31 content in women was 10.3 nmol / L. The CD31 content was 29 nmol / L in menopausal aged mastitis patients. Women with menopause age FCM were found to have CD31 levels 2.8 times lower when taken compared to inflammation. In menopausal age fibroadenoma patients, the amount of CD31 in women was 2 nmol/L, and in menopausal age mastitis patients, the amount of CD31 in women was 29 nmol/L. The amount of CD31 in patients with fibroadenoma was found to be 14.5 times lower than in patients with inflammatory disease (Figure 1).
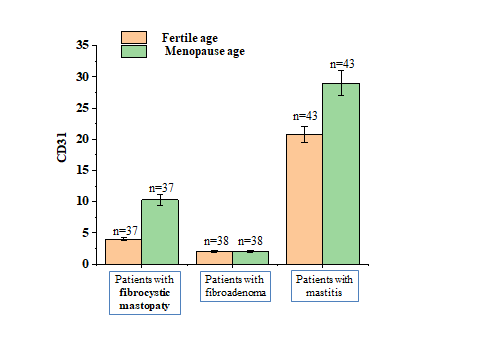 | Figure 1. Changes in CD31 levels in healthy women of fertile and menopausal age and in precancerous breast tumors |
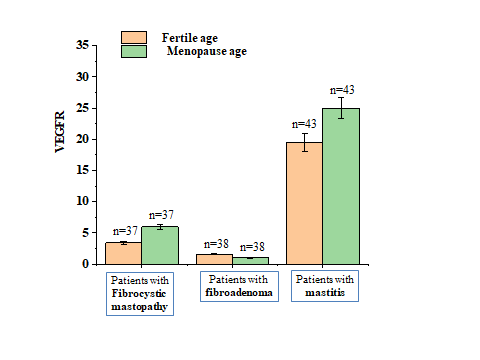 | Figure 2. Changes in VEGFR levels in healthy women of fertile and menopausal age and in breast precancer |
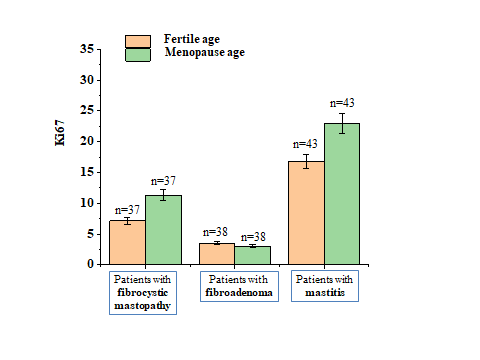 | Figure 3. Changes in Ki67 levels in healthy women of fertile and menopausal age and in breast precancer |
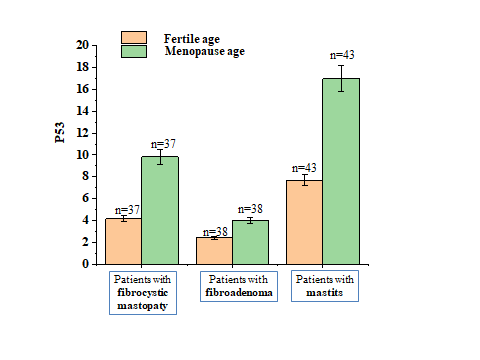 | Figure 4. Changes in P53 levels in healthy and premenopausal women of fertile age and in women with precancerous breast cancer |
4. Conclusions
- In the analysis of the tumor microenvironment in pre-cancer cases, a comprehensive assessment of these markers allows us to determine the biological activity of the tumor microenvironment, select individual treatment for patients, and perform early diagnosis. The interaction of CD31, VEGFR, Ki-67, and p53 markers in the tumor microenvironment plays an important role in the early development of breast cancer and its effective treatment.Therefore, a deep study of the biological mechanisms of the tumor microenvironment, the identification of their early diagnostic biomarkers, and the development of therapeutic strategies aimed at stromal and immune components are among the most promising areas in the prevention and early diagnosis of breast cancer. Future studies are expected to expand the possibilities of stopping or reversing malignant transformation by modulating the microenvironment.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML