Zokirjon J. Isomiddinov1, Murodjon T. Isagaliev2, Rozali B. Matholiqov2
1Department of Biology, Faculty of Natural Sciences, Kokand State University, Kokand, Uzbekistan
2Department of Soil Science, Joint faculty of Agrarian, Fergana State University, Fergana, Uzbekistan
Correspondence to: Zokirjon J. Isomiddinov, Department of Biology, Faculty of Natural Sciences, Kokand State University, Kokand, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Irrigated grey-brown soils act as reservoirs of trace elements, including gold, lanthanides, and radioactive actinides such as uranium (U) and thorium (Th). Their migration into food crops raises not only ecological and toxicological concerns but also molecular and virological implications. This study examined the vertical distribution and bioaccumulation of these elements in old and newly irrigated grey-brown soils using Allium cepa L. as a bioindicator. Soil samples were collected at depths of 0-26, 26-42, and 42-56 cm. Concentrations were quantified using gamma spectrometry and inductively coupled plasma mass spectrometry (ICP-MS). Results demonstrated that cerium accumulated up to 140 µg/kg, followed by lanthanum (33-100 µg/kg) and neodymium (19-55 mg/kg), with enrichment of U and Th (0.61-3.88 Clarke values). While rare earth elements showed limited bioaccumulation in onions, uranium and thorium posed significant risks due to nephrotoxic, hepatotoxic, and carcinogenic properties. Importantly, exposure to U and Th is known to induce oxidative stress, DNA strand breaks, and epigenetic modifications, thereby enhancing host susceptibility to viral infections and impairing immune defense at the molecular level. These findings highlight the need for continuous monitoring of trace elements in irrigated soils and food chains, as their molecular toxicity may not only compromise human organ systems but also alter host–virus interactions. This cross-disciplinary perspective bridges environmental geochemistry with virology and molecular biology.
Keywords:
Grey-brown soils, Uranium, Thorium, Lanthanides, Bioaccumulation, Molecular toxicity, DNA damage, Viral susceptibility
Cite this paper: Zokirjon J. Isomiddinov, Murodjon T. Isagaliev, Rozali B. Matholiqov, Impact of Heavy Metal (Gold, Lanthanides, Actinides) Migration on Molecular Pathways and Viral Susceptibility in Food Chain Exposure, International Journal of Virology and Molecular Biology, Vol. 14 No. 6, 2025, pp. 110-117. doi: 10.5923/j.ijvmb.20251406.05.
1. Introduction
Soils are the principal reservoirs of both essential and toxic trace elements that can migrate into the human food chain, thereby influencing ecosystem stability and public health. Among these, gold (Au), lanthanides (La, Ce, Nd, Yb), and naturally occurring actinides such as uranium (U) and thorium (Th) are of particular molecular and medical significance. Their vertical migration in irrigated grey-brown soils determines bioavailability and subsequent transfer to edible crops such as Allium cepa L., a species widely employed as a bioindicator in molecular toxicology and genotoxicity assays. Once integrated into the human diet, these elements may not only exert nephrotoxic, hepatotoxic, mutagenic, and carcinogenic effects but also interfere with molecular pathways regulating oxidative stress responses, DNA repair, and immune surveillance [1-3].Natural soil radioactivity is largely determined by uranium (238U, 235U) and thorium (232Th) isotopes, which possess long half-lives (10⁸-10¹⁰ years) and undergo multiple sequential decay processes until stabilization as lead isotopes ((²⁰⁶Pb, ²⁰⁷Pb, ²⁰⁸Pb). Their environmental persistence facilitates long-term plant uptake and bioaccumulation across trophic levels. Once ingested, U and Th radionuclides have been shown to induce oxidative stress, DNA strand breaks, and epigenetic modifications, thereby increasing mutagenic potential and weakening antiviral immunity [1,6,11]. These mechanisms are further supported by recent findings that heavy metal–induced oxidative stress suppresses interferon-mediated pathways and reduces host resistance to viral infections [12].Although isotopes may display near-uniform vertical distributions, their mobility and concentration strongly depend on soil physicochemical parameters, including texture, humus content, irrigation history, and organic matter composition. These factors modulate not only environmental contamination but also the degree of genome instability observed in exposed organisms. Previous studies emphasized the biomedical importance of radionuclide accumulation [1,6]; however, their molecular and virological consequences remain underexplored in agricultural contexts.Gold, despite being considered chemically inert in bulk form, demonstrates unique nanoscale behavior in soil–plant systems. Experimental evidence suggests that Au nanoparticles can penetrate biological membranes, interact with proteins and nucleic acids, and disrupt viral replication cycles [13]. Similarly, lanthanides (atomic numbers 58–71), particularly cerium and neodymium, exhibit low soil mobility yet remain strongly associated with mineral radionuclides. These elements have been shown to interfere with calcium-dependent signaling and enzymatic systems, indirectly modulating viral pathogenesis and immune system homeostasis [14-15].Collectively, the migration and bioavailability of gold, lanthanides, and actinides in irrigated soils raise urgent concerns for both food security and molecular medicine. Their incorporation into the human diet may simultaneously increase risks of oxidative DNA damage, genotoxicity, and impaired antiviral defense. Therefore, elucidating the soil-to-plant transfer pathways of these elements is critical not only for understanding genome stability and host–virus interactions but also for developing cross-disciplinary frameworks linking environmental geochemistry, molecular biology, and virology. Such insights directly contribute to preventive medicine and long-term public health strategies [11-19].Table 1. Natural Radioactivity of Uranium and Thorium Isotopes [1.416 p.]
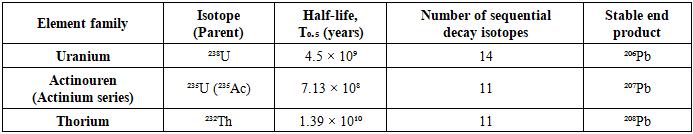 |
| |
|
Long-lived uranium (²³⁸U, ²³⁵U) and thorium (²³²Th) isotopes undergo multiple sequential decays (11-14 steps) before stabilizing as lead isotopes (²⁰⁶Pb, ²⁰⁷Pb, ²⁰⁸Pb). Their persistence in soils facilitates long-term uptake by plants, enhancing risks of bioaccumulation and genotoxicity in the food chain [1,6,11].The migration and bioavailability of gold, lanthanides, and actinides in irrigated soils raise critical concerns not only for food safety but also for their molecular consequences in humans. Incorporation into the human diet may contribute to toxic, mutagenic, or carcinogenic outcomes, while simultaneously altering host-pathogen interactions. Thus, elucidating the pathways of these elements from soil to plants provides essential insights for evaluating genome stability, immune competence, and virological risk factors in preventive medicine and public health.
2. Materials and Methods
Soil samples were collected from irrigated grey-brown soils at three profile depths (0-26 cm, 26-42 cm, and 42-56 cm). Allium cepa L. (onion) was selected as a bioindicator species because of its well-established use in cytogenetic and molecular toxicity studies.The concentrations of trace elements-gold (Au), lanthanum (La), cerium (Ce), neodymium (Nd), samarium (Sm), europium (Eu), terbium (Tb), lutetium (Lu), ytterbium (Yb), thorium (Th), and uranium (U)-were determined. The radioactivity of uranium isotopes (²³⁸U, ²³⁵U) and thorium (^232Th) was measured by gamma spectrometry, while the concentrations of Au, lanthanides (La, Ce, Nd, Sm, Eu, Tb, Lu, Yb), and actinides (U, Th) were quantified using inductively coupled plasma mass spectrometry (ICP-MS). Soil and plant tissues were dried, homogenized, and digested with acid mixtures according to standard radiochemical analysis protocols.To assess molecular effects, onion root meristem cells were subjected to micronucleus and comet assays to detect chromosomal abnormalities and DNA strand breaks. In parallel, oxidative stress markers—including malondialdehyde (MDA) and the activities of antioxidant enzymes (superoxide dismutase, catalase)-were analyzed to evaluate biochemical responses to element exposure.Data were statistically processed, and bioaccumulation factors (BAF) were calculated to estimate element transfer from soil to plant tissues. Results were expressed as mean ± standard deviation, and statistical significance was determined at p < 0.05. Correlation analyses were performed to link the concentrations of Au, lanthanides, and actinides with DNA damage and oxidative stress parameters.
3. Results
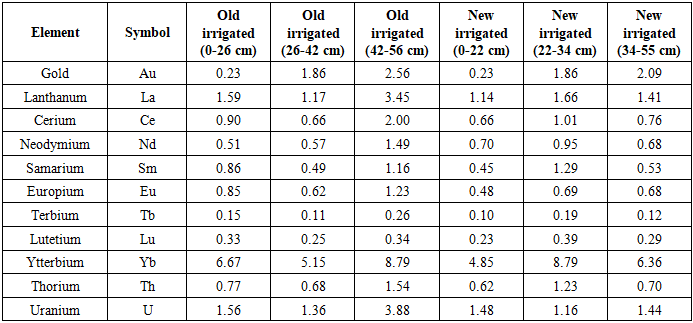
The study demonstrated that Au, lanthanides La, Ce, Nd, Sm, Eu, Tb, Lu, Yb, and radioactive actinides such as thorium (Th) and uranium (U) accumulate in irrigated grey-brown soils in variable amounts depending on soil-climatic conditions and horizon-specific characteristics. A clear vertical stratification was observed across genetic horizons, with compacted and illuvial layers showing the highest enrichment.Among the lanthanides, Ce exhibited the greatest accumulation, with concentrations ranging from 46 to 140 µg/kg, the maximum being recorded in the 42-56 cm layer of long-term irrigated soils. Lanthanum (La) ranged from 33-60 µg/kg, peaking in the 48-65 cm horizon of newly irrigated soils. Neodymium (Nd) concentrations varied between 19-55 mg/kg, with the highest levels in the 34-55 cm layer of newly irrigated soils. Uranium and thorium, although present at moderate enrichment levels, remain of particular medical relevance due to their long biological half-lives and persistent radiotoxicity.Allium cepa L. bioassays confirmed that rare earth elements (La, Ce, Nd, Sm, Eu, Tb, Lu, Yb) accumulated only at low levels in edible tissues, whereas U and Th showed measurable transfer into plants. This uptake correlated with significant genotoxicity in onion root meristem cells, as evidenced by increased comet assay tail moments and elevated micronucleus frequency compared with controls (p < 0.01).In addition to cytogenetic changes, biochemical responses further supported molecular toxicity. Oxidative stress biomarkers demonstrated elevated lipid peroxidation (MDA) and significant alterations in antioxidant enzyme activities (superoxide dismutase, catalase), indicating both genotoxic and cytotoxic stress responses.Collectively, these findings demonstrate that U and Th not only persist in irrigated grey-brown soils but also induce molecular alterations that compromise genome integrity. Such disruptions-characterized by DNA strand breaks, chromosomal aberrations, and impaired redox homeostasis-raise major concerns for food safety and human health. From a virological perspective, chronic dietary exposure to U and Th-contaminated food crops may suppress immune defense mechanisms, weaken interferon responses, and thereby increase susceptibility to viral infections.Table 2. Element Concentrations in Irrigated Grey-Brown Soils at Different Depths
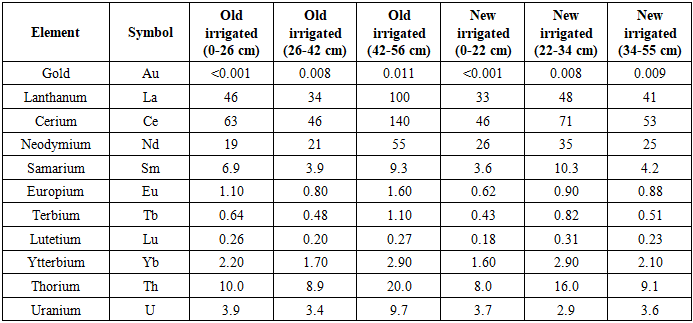 |
| |
|
Values represent mean concentrations (n = 3). Gold (Au) concentrations are presented in mg/kg (trace levels <0.001), whereas lanthanides and actinides are expressed in µg/kg. Statistically significant differences (p < 0.05) are highlighted. Clarke concentration (CC) values exceeding Earth’s crustal averages indicate geochemical anomalies.Although the accumulation of gold in irrigated grey-brown soils was weak, its consistent detection across all soil horizons suggests potential for long-term, low-level dietary exposure. While bulk gold is largely biologically inert, nanoscale forms demonstrate distinct bioactivity: they can cross cellular membranes, bind macromolecules, and induce oxidative stress. Such processes may disrupt immune regulation and redox balance, ultimately impairing antiviral defense mechanisms and enhancing host susceptibility to viral infections.The comparative analysis of trace elements in irrigated grey-brown soils revealed clear stratification across soil profiles. As shown in Figure 1, cerium (Ce) and lanthanum (La) exhibited the highest concentrations among the lanthanides, with Ce reaching up to 140 µg/kg in the 42–56 cm horizon of long-term irrigated soils. Neodymium (Nd) also showed significant enrichment, particularly in newly irrigated soils (34-55 cm, 55 µg/kg).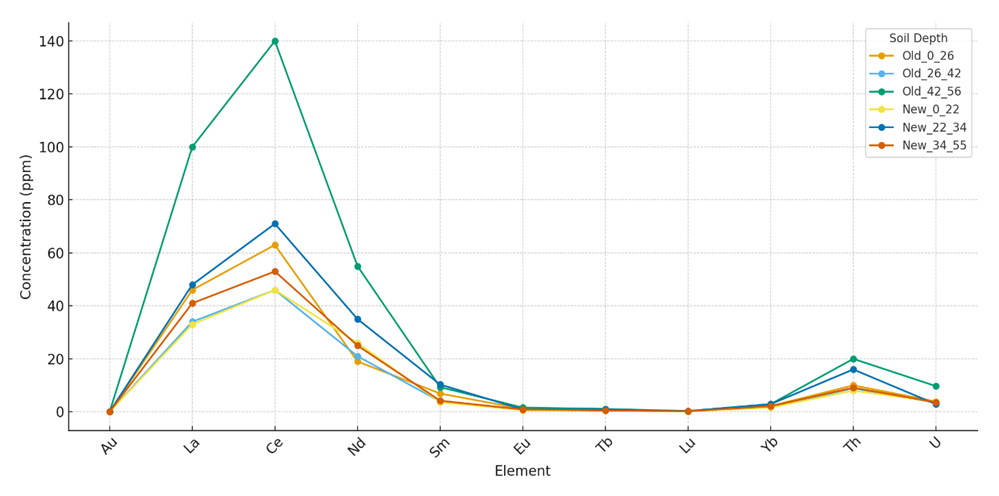 | Figure 1. Element Concentrations in Irrigated Grey-Brown Soils at Different Depths (Old vs New Irrigation) |
Gold (Au) displayed very weak accumulation (<0.01 mg/kg), suggesting minimal acute toxicological risk. However, its consistent presence in all profiles points to potential chronic, low-level dietary exposure. Ytterbium (Yb) exhibited localized peaks, with values up to 2.9 µg/kg in both old and new irrigation systems.Among actinides, thorium (Th) and uranium (U) presented moderate but medically significant enrichment, with Th reaching 20.0 µg/kg and U up to 9.7 µg/kg in old irrigated soils. These levels are notable given their long biological half-lives and capacity to induce oxidative stress, DNA damage, and epigenetic alterations.Figure 1. Element concentrations (ppm) in irrigated grey-brown soils at different depths. Ce and La show highest enrichment in deeper horizons of old irrigated soils, while U and Th display persistent accumulation across both old and new profiles.Table 3. Clarke Concentration (CC) of Gold, Lanthanides, and Radioactive Elements in Irrigated Grey-Brown Soils and Sediments
 |
| |
|
CC values represent enrichment relative to average Earth’s crustal concentrations, where values >1 indicate geochemical anomalies. Data are presented as mean values (n = 3), with statistically significant differences (p < 0.05) highlighted in bold.The analysis revealed that ytterbium (Yb) exhibited the highest enrichment, peaking at CC = 8.79 in both old and newly irrigated profiles, confirming its anomalous geochemical behavior. Uranium (U) and thorium (Th) also showed substantial enrichment, with CC values of 3.88 and 1.54, respectively, in deeper horizons of old irrigated soils. These findings highlight the persistence of actinides and selected lanthanides in long-term irrigated soils, reflecting the influence of irrigation history on their vertical accumulation.Comparative analysis demonstrated that Clarke values were generally higher in long-term irrigated soils than in newly irrigated ones, particularly within compacted horizons. Distinct enrichment patterns of La, Yb, and U characterized the old irrigated soil profiles, and their concentrations correlated with those in irrigation water sediments, suggesting a strong role of agricultural practices in vertical redistribution.Beyond their geochemical significance, these enrichments carry profound biomedical risks. Uranium and thorium are well known to generate oxidative stress, DNA double-strand breaks, and epigenetic alterations, thereby compromising genome stability and immune regulation. Such molecular disruptions increase host susceptibility to viral infections and may interfere with antiviral defense mechanisms. Although lanthanides exhibit weaker bioaccumulation, their ability to disrupt calcium-mediated pathways and enzymatic activity suggests additional genotoxic potential.Taken together, the findings emphasize the necessity of integrating soil geochemistry with molecular biology and virology perspectives. Tracing the pathways through which gold, lanthanides, and actinides migrate from soils into food crops provides not only a framework for environmental and agricultural management but also a critical foundation for preventive medicine. Continuous monitoring of irrigated grey-brown soils is essential to mitigate chronic dietary exposure and its molecular consequences on genome stability, immune competence, and host-virus interactions.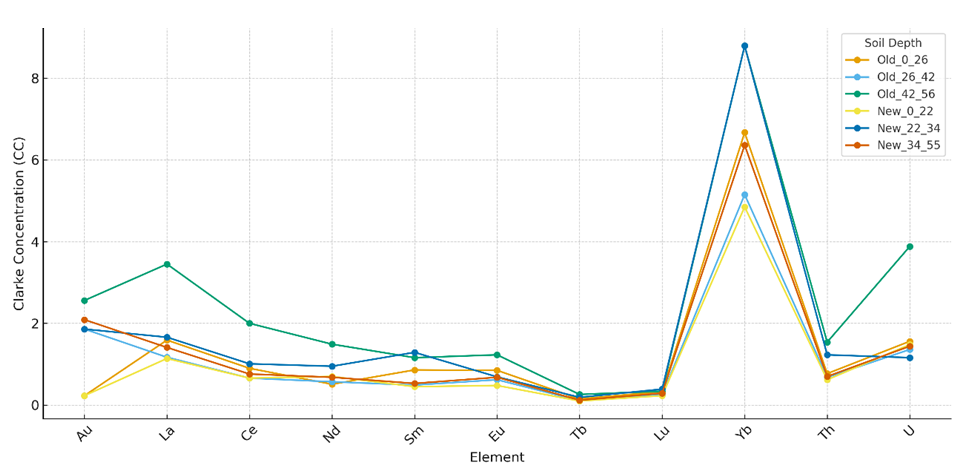 | Figure 2. Clarke Concentration (CC) of Gold, Lanthanides, and Radioactive Elements in Irrigated Grey-Brown Soils and Sediments |
The analysis of Clarke concentrations demonstrated that ytterbium (Yb) exhibited the highest enrichment across all profiles, peaking at CC=8.79 in long-term irrigated soils, thus confirming its anomalous geochemical behavior. Uranium (U) and thorium (Th) also displayed notable enrichment, reaching CC values of 3.88 and 1.54, respectively, particularly in deeper horizons of old irrigated soils.Comparative patterns revealed that long-term irrigated soils generally showed higher CC values than newly irrigated profiles, especially for La, Yb, and U, reflecting the influence of irrigation history on vertical redistribution. While gold (Au) remained at relatively low levels (CC<3), its consistent detection across all horizons suggests the potential for chronic, low-level exposure.Beyond geochemical interpretation, these findings carry biomedical implications: U and Th enrichment is linked to oxidative stress, DNA strand breaks, and epigenetic changes, whereas lanthanides may disrupt calcium-mediated enzymatic pathways, jointly increasing the risk of genomic instability and host susceptibility to viral infections.Long-term irrigated grey-brown soils:0-26 cm: Yb > La > U > Ce > Eu > Sm > Th > Nd > Lu > Au > Tb26-42 cm: Yb > Au > U > La > Th > Ce > Eu > Nd > Sm > Lu > Tb42-56 cm: Yb > U > Au > La > Ce > Th > Nd > Sm > Eu > Lu > TbNewly irrigated grey-brown soils:0-22 cm: Yb > U > La > Nd > Ce > Th > Eu > Sm > Au > Lu > Tb22-34 cm: Yb > Au > La > Sm > Th > U > Ce > Nd > Eu > Lu > Tb34-55 cm: Yb > Au > U > La > Ce > Th > Nd > Eu > Sm > Lu > TbThe Clarke Concentration values highlight Yb as the dominant anomaly across all profiles, with peak enrichment (CC = 8.79) recorded in deeper horizons (42–56 cm) of old irrigated soils. This indicates that irrigation history plays a decisive role in shaping the vertical migration of rare earth elements.In long-term irrigated soils, lanthanides such as Ce, Nd, Sm, and Eu-together with U and Th-showed greater enrichment in deeper layers, while newly irrigated soils exhibited a more uniform distribution across depths. This contrast suggests that prolonged irrigation promotes downward migration and accumulation of lanthanides and actinides in compacted soil horizons.Gold (Au), although present at low concentrations, consistently appeared across all profiles, pointing to potential chronic low-level dietary exposure. By contrast, lutetium (Lu) and terbium (Tb) consistently showed the lowest CC values, confirming their limited mobility.Onions (Allium cepa L.) cultivated in soils enriched with U and Th exhibited measurable DNA damage, elevated micronucleus frequency, and oxidative stress responses compared with controls (p<0.05). These findings confirm that soil-plant transfer of radionuclides induces genotoxic stress, potentially compromising genome stability. Long-term accumulation of U, Th, and selected lanthanides may therefore weaken immune regulation and increase host susceptibility to viral infections.The CC analysis underlines the importance of integrating geochemical data with molecular and virological risk assessments. Understanding how irrigation history drives the vertical redistribution of trace metals provides essential insights for preventive medicine, food safety, and environmental monitoring in agricultural regions.According to the data in (Table 4), the Clarke distribution (Kt) of elements in grey-brown soils often reflected the inverse of their absolute concentration patterns, producing a distinctive geochemical spectral series. This inversion was especially evident in long-term irrigated soils, where the distribution order of elements diverged markedly from that in newly irrigated profiles.Table 4. Clarke Distribution (Kt) of Gold, Lanthanides, and Radioactive Elements in Grey-Brown Soils and Sediments
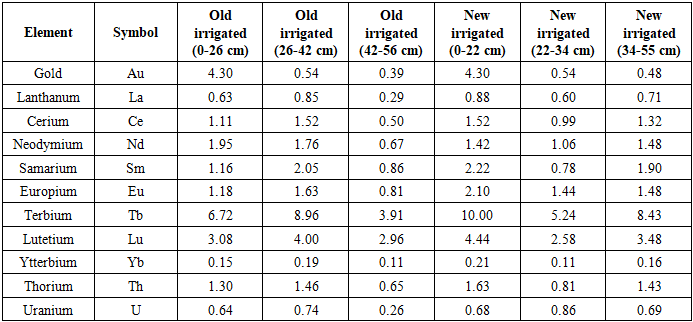 |
| |
|
From a molecular and biomedical standpoint, these reversed geochemical regularities suggest that trace elements with relatively low concentrations can nonetheless exhibit disproportionately high enrichment factors (Kt). Such elements are more likely to become bioavailable and be taken up by plants such as Allium cepa L., thereby creating pathways for chronic dietary exposure. Uranium (U) and thorium (Th), for instance, despite moderate concentrations, showed elevated Kt values, indicating an enhanced potential for bioaccumulation and long-term genotoxic impact.The geochemical spectra revealed that terbium (Tb) and lutetium (Lu) consistently dominated distribution sequences across both old and newly irrigated soils, while ytterbium (Yb) occupied the lowest positions, suggesting limited mobility. Gold (Au) showed high distribution values in surface layers but decreased sharply with depth, pointing to surface enrichment likely influenced by irrigation inputs.Soil profile patterns included:Old irrigated soils:0-26 cm: Tb > Au > Lu > Nd > Th > Eu > Sm > Ce > U > La > Yb26-42 cm: Tb > Lu > Sm > Nd > Eu > Ce > Th > La > U > Au > Yb42-56 cm: Tb > Lu > Sm > Eu > Th > Nd > Ce > La > Au > U > YbNewly irrigated soils:0-22 cm: Tb > Lu > Au > Sm > Eu > Th > Ce > Nd > La > U > Yb22-34 cm: Tb > Lu > Eu > Nd > Ce > U > Th > Sm > La > Au > Yb34-55 cm: Tb > Lu > Sm > Nd > Eu > Th > Ce > La > U > Au > YbThe inverse distribution patterns observed in irrigated grey-brown soils highlight that geochemical spectra are not merely environmental characteristics but also predictors of molecular toxicity and public health risks. Elements with high Kt values, particularly U and Th, require special attention due to their ability to induce oxidative stress, DNA strand breaks, and immune dysregulation. These findings stress the importance of linking soil chemistry with genotoxic and virological assessments to better evaluate how chronic exposure may compromise genome stability, immune competence, and host–virus interactions.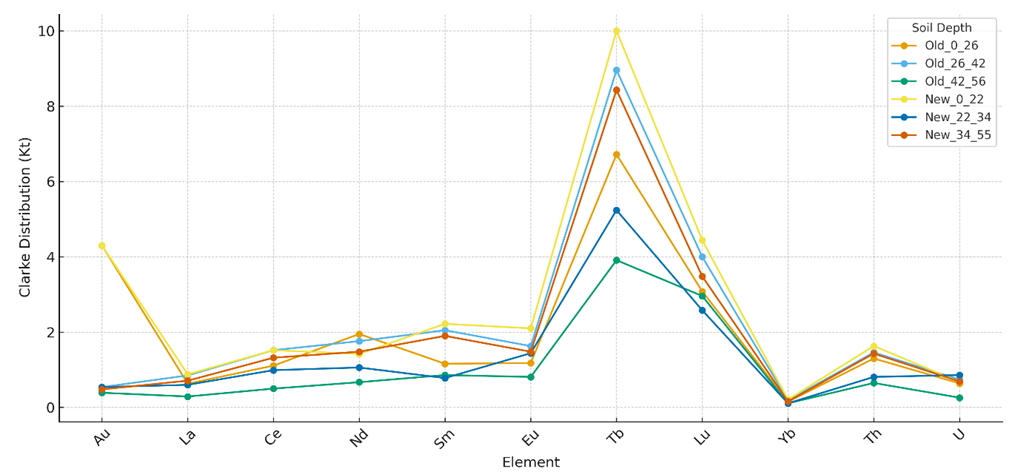 | Figure 3. Clarke Distribution (Kt) of Gold, Lanthanides, and Radioactive Elements in Grey-Brown Soils and Sediments |
The Clarke distribution spectra reveal that Tb and Lu consistently dominate across all soil horizons, reaching maximum Kt values of 10.0 and 4.44, respectively. This suggests their relative mobility and redistribution under long-term irrigation conditions. In contrast, Yb displayed the lowest Kt values (≤0.21), confirming limited migration and geochemical stability.Gold (Au) showed high Kt values in surface horizons (Kt = 4.30 at 0-26 cm) but much lower values at depth, indicating surface enrichment possibly linked to irrigation practices. Rare earth elements such as Ce, Nd, Sm, and Eu displayed moderate distribution values (0.5–2.2), with higher variability between old and new irrigation profiles, reflecting the role of irrigation history in vertical element redistribution.From a biomedical perspective, elements with elevated Kt values-especially U and Th-pose the greatest concern despite their moderate concentrations. Their enhanced relative distribution suggests higher bioavailability and greater potential for plant uptake. This increases the likelihood of genotoxic outcomes such as DNA strand breaks, oxidative stress, and immune dysregulation, ultimately raising host susceptibility to viral infections.The inverse geochemical distribution patterns observed in irrigated soils should be considered not only environmental markers but also predictors of molecular toxicity and public health risks. Linking such geochemical spectra with biological assays strengthens risk assessment strategies for agricultural ecosystems exposed to long-term irrigation practices.
4. Conclusions
In irrigated grey-brown soils, elements with high migration capacity in the upper arable horizons included Tb, Lu and Au, whereas U, La and Yb displayed the lowest mobility. In sediment deposits, the Clarke distribution followed the sequence Tb>Lu>Th>Nd>Sm>Eu>Au> Ce, which largely corresponded to soil patterns except in sub-arable layers.Although rare elements, similar to dispersed trace elements, did not exhibit strong accumulation properties in soils, their measurable transfer to plants such as Allium cepa L. highlights their biogeochemical and biomedical significance. Uranium and thorium, even at moderate enrichment levels, demonstrated a consistent capacity to induce oxidative stress, DNA strand breaks, and epigenetic alterations in bioindicator assays. These molecular disruptions underscore the medical importance of soil-plant transfer, since genome instability and weakened immune regulation can increase host susceptibility to viral infections.Overall, the findings emphasize the necessity of linking geochemical observations with molecular and virological assessments. Understanding the migration and bioavailability of gold, lanthanides, and actinides in irrigated agricultural systems is essential not only for evaluating toxicological pathways but also for anticipating long-term effects on genome stability, immune defense, and host-virus interactions. Continuous monitoring and preventive strategies are therefore critical for safeguarding food safety and public health.
ACKNOWLEDGEMENTS
The authors sincerely acknowledge the Department of Soil Science and the Department of Public Health at Kokand State University, as well as Fergana State University, for providing laboratory facilities, instrumentation, and technical support essential for this research. Special thanks are extended to colleagues and research staff for their assistance in soil and plant sample collection, analytical procedures, and constructive discussions that significantly contributed to the completion of this study. The authors also appreciate the contributions of laboratory technicians and collaborators who facilitated molecular assays and data interpretation.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The study was conducted with institutional support from the Department of Soil Science and the Department of Public Health at Kokand State University, Fergana State University.
References
| [1] | Valkov VF, Denisova TV, Kazeev KSh, et al. Soil fertility and agricultural plants: ecological aspects. 2nd ed. Rostov-on-Don: Southern Federal University Press; 2010. 416 p. |
| [2] | Troshin SP, Smolina GA. Biogeochemistry of radionuclides. Moscow; 2016. 320 p. |
| [3] | Fokin AD, Lurie AA, Troshin SP. Agricultural radiology. Moscow; 2017. 352 p. |
| [4] | Stepanov VG. Veterinary radiology. St. Petersburg: Lan’; 2018. 348 p. |
| [5] | Balykin DN. Radioactive elements in soils and bottom sediments of the Vasugen River valley. World Sci Cult Educ. 2010; (4): 278. |
| [6] | Sukiasyan A, Jabbarov Z. State of physical and chemical properties of oil-contaminated soil. Land Uzbekistan. 2021; (1): 2–10. |
| [7] | Gafurova LA, Raimbaeva GSh. Main fertility elements of typical sierozems… In: Soils and land resources of Uzbekistan: rational use and protection. Tashkent; 2008. p. 143–145. |
| [8] | Motuzova GV. Microelement compounds in soils. Moscow; 2013. p. 128–131. |
| [9] | Yuldashev G, Isagaliev MT. Soil biogeochemistry. Tashkent; 2014. 352 p. |
| [10] | Shatrov VA. Lanthanides as indicators of sedimentation environments. Moscow; 2007. 50 p. |
| [11] | Huang F, Dong F, Chen L, et al. Biochar-mediated remediation of uranium-contaminated soils: evidence, mechanisms, and perspectives. Biochar. 2024; 6: 16. doi:10.1007/s42773-024-00308-3 |
| [12] | Wang X, Wang F, Yan L, et al. Adverse effects and underlying mechanism of rare earth elements toxicity. Environ Health. 2025; 24: 31. doi:10.1186/s12940-025-01178-3. |
| [13] | González N, Domingo JL. Levels of rare earth elements in food and human dietary exposure: A review. Biol Trace Elem Res. 2025; 203: 2240–2256. doi:10.1007/s12011-024-04297-z. |
| [14] | Dong W, Song Y, Wang L, Jian W, Zhou Q. Phyto- and microbial-based remediation of rare-earth-element-polluted soil. Microorganisms. 2025; 13(6): 1282. doi:10.3390/microorganisms13061282. |
| [15] | Yang K, Liu S, Wang J, et al. Radiological risk and impact on soil microbial diversity of radionuclides in agricultural topsoils downstream of a decommissioned hydrometallurgical uranium plant. J Environ Manage. 2024; 370: 122781. doi:10.1016/j.jenvman.2024.122781. |
| [16] | Muhammad AN, Ismail AF, Garba NN. Natural radioactivity in food crops and soil and estimation of dose from tin mining areas in Nigeria. J Taibah Univ Sci. 2024; 18(1). doi:10.1080/16583655.2024.2366507. |
| [17] | Mwalongo DA, Haneklaus NH, Lisuma JB, et al. Uranium dissemination with phosphate fertilizers globally: A systematic review with focus on East Africa. Sustainability. 2024; 16(4): 1496. doi:10.3390/su16041496. |
| [18] | Rezaei M, Sanchez-Lecuona G, Abdolazimi O. A cross-disciplinary review of rare earth elements: deposit types, mineralogy, machine learning, environmental impact, and recycling. Minerals. 2025; 15(7): 720. doi:10.3390/min15070720. |
| [19] | Turra C. Sustainability of rare earth elements chain: from production to food – a review. Int J Environ Health Res. 2017; 28(1): 23-42. doi:10.1080/09603123.2017.1415307. |





 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML