-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Virology and Molecular Biology
p-ISSN: 2163-2219 e-ISSN: 2163-2227
2025; 14(6): 107-109
doi:10.5923/j.ijvmb.20251406.04
Received: Aug. 29, 2025; Accepted: Sep. 25, 2025; Published: Sep. 29, 2025

Antibacterial and Antifungal Activity of Certain Lactobacillus Species Against Conditional Pathogenic and Pathogenic Microorganisms
Temirova Munira Baxriddinova1, Tashbaev Sherzodbek Abdurasulovich2
1PhD Student, Department of Biology, Andijan State University, Uzbekistan
2PhD Associate Professor, Department of Biology, Andijan State University, Uzbekistan
Correspondence to: Temirova Munira Baxriddinova, PhD Student, Department of Biology, Andijan State University, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

According to the results of the study, 4 strains showed high antifungal activity against the selected phytopathogenic micromycetes Alternaria alternate, Verticillium dahlia, Aspergillus flavus, Aspergillus oryzae and Fusarium specius. The antifungal activity of the bacterial strain L. fermentum No. 1 against the phytopathogenic Alternaria alternate, Verticillium dahlia, Aspergillus oryzae and Fusarium specius was determined in the zones of 27±0.3, 15±0.3, 19.6±1.5 and 18±0.6 mm. The antifungal activity of lactic acid bacteria strains was determined by the growth radius of the phytopathogens and it was found that they form a ring from 15 to 35 mm.
Keywords: Lactobacillus plantarum, L. brevis, L. fermentum, Pediococcus pentosaceus, Phytopathogen, Antibacterial, Antifungal property, Phytopathogen, Strain, Lactic acid bacteria
Cite this paper: Temirova Munira Baxriddinova, Tashbaev Sherzodbek Abdurasulovich, Antibacterial and Antifungal Activity of Certain Lactobacillus Species Against Conditional Pathogenic and Pathogenic Microorganisms, International Journal of Virology and Molecular Biology, Vol. 14 No. 6, 2025, pp. 107-109. doi: 10.5923/j.ijvmb.20251406.04.
1. Introduction
- The anticipated population growth in the coming decades and the increasing demand for livestock products necessitate the production of larger quantities of food products. Concerns about food quality in industrialized countries and the need to enhance soil fertility are among the major challenges facing global agriculture today [1]. The isolation and study of probiotic bacterial strains, as well as the development of new applications in this field, hold significant importance. The use of probiotics in crop production is gradually gaining traction [2]. The isolation of local microorganism strains with high antagonistic properties and the identification of their biologically active compounds are of great significance [3]. When discussing the biological activity of bacteria, it can be noted that they are completely safe for humans and the environment. Their culture supernatant has the ability to produce signaling molecules, immunoactive proteins, and biologically active compounds, including not only peptide antibiotics but also hormone-like substances that play a special role [4,5]. Additionally, probiotics produce substances (e.g., organic acids, peptides, cyclic dipeptides, fatty acids, volatile compounds, lactic acid, bacteriocins, hydrogen peroxide, and antibiotics) that exhibit activity against pathogenic and conditionally pathogenic microorganisms [6]. At present, plant fungal diseases are primarily controlled using synthetic fungicides. However, their excessive and improper use harms the environment and increases the resistance of phytopathogens to these fungicides [7].Under the conditions of Uzbekistan, four strains of lactic acid bacteria were isolated from locally sourced cow milk yogurt and pickled cabbage: Lactobacillus plantarum №1, Lactobacillus brevis №1, Lactobacillus fermentum №1, Pediococcus pentosaceus №1.These strains were identified based on their morphological and cultural characteristics using Bergey's Manual of Determinative Bacteriology, as well as by MALDI-TOF mass spectrometry. The antagonistic properties of the lactic acid bacteria isolated from pickled cabbage and kefir were determined using the agar block method. Selected lactic acid bacteria were cultivated on MRS agar medium (HiMedia, pH 6.2) in Petri dishes for 48 hours at room temperature. Phytopathogenic micromycetes were cultured on solid Czapek-Dox agar medium (g/l: KH₂PO₄ – 1.0; MgSO₄ – 0.5; NaNO₃ – 3.0; KCl – 0.5; FeSO₄; sucrose – 2.0; trace elements – 1 ml; trace element mixture: 500 mg FeSO₄, 156 mg MnSO₄·4H₂O, 167 mg ZnCl₂, 200 mg CoCl₂, 1 ml 19% HCl in 100 ml distilled water) for 6 days at 28°C. 6 mm wells were made in the Petri dishes containing the lactic acid bacteria cultures. A spore suspension of phytopathogenic fungi was prepared at a concentration of 10⁷ spores/ml from 4-day-old cultures grown on Czapek agar. Lactobacillus plantarum demonstrated strong antifungal activity against phytopathogenic micromycetes from the genera Aspergillus, Fusarium, Penicillium, and Rhizopus [8]. To assess bacteriocinogenic activity, the cultures were inoculated into MRS medium and incubated at 37±1°C for 24 hours. Then, 1.5% agar-containing Petri dishes were prepared, and marked according to the number of test strains. 2–5 µl of 12-hour bacterial cultures were spotted on each mark. The inoculated dishes were incubated at 37±1°C for 4–12 hours. Subsequently, the cultures were exposed to ultraviolet radiation for 30 seconds, followed by an additional 3–12 hours of incubation under the same conditions. The cultures were grown for 48 hours and then treated with chloroform. The Petri dishes were ventilated for 15 minutes. Thereafter, 3–5 ml of test cultures with a titer of 10⁷ CFU/ml were inoculated into a soft agar overlay (0.7%) and incubated at 37±1°C for 4–12 hours. The antimicrobial activity of the lactic acid bacterial cultures was studied against the following seven opportunistic pathogens: Aeromonas veronii, Staphylococcus aureus, Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa, Proteus mirabilis, Candida albicans.
2. Materials and Methods
- Lactobacillus brevis №1 showed strong antibacterial activity against St. aureus, B. subtilis, E. coli, and C. albicans, but had no activity against P. aeruginosa. Lactobacillus fermentum №1 exhibited the highest antimicrobial activity against St. aureus, E. coli, Pr. mirabilis, and C. albicans. Pediococcus acidilactici P1 showed strong activity against E. coli, P. aeruginosa, and C. albicans. L. plantarum and L. fermentum showed no antimicrobial activity against A. veronii. (Figure 1)
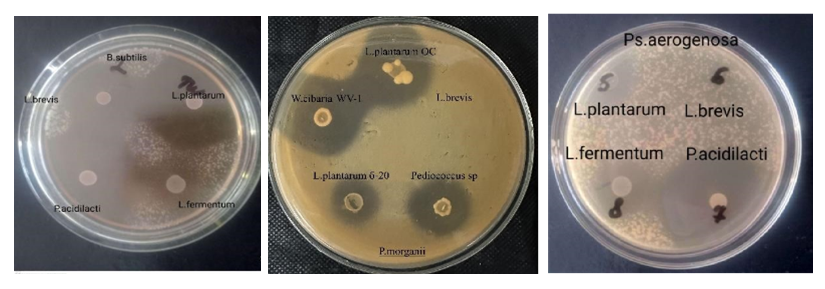 | Figure 1. Antibacteriocinogenic Activity of Lactic Acid Bacteria |
3. Results and Analysis
- To determine the antibiotic sensitivity of Lactobacillus strains, the disk diffusion method was employed. Selected Lactobacillus strains were grown in MRS nutrient medium at 37°C for 24 hours. The cell suspension was adjusted to a concentration of 10⁷ CFU/ml, and uniformly spread on the surface of MRS agar using a sterile spreader.Antibiotic discs were placed on the surface of the inoculated Petri dishes, containing the following antibiotics: Rifampicin (5 µg), Erythromycin (15 µg), Oxacillin (1 µg), Cefotaxime (30 µg), Gentamicin (10 µg), Cefazolin (30 µg), Tetracycline (30 µg) The plates were incubated at 37°C for 24 hours. The diameter of the inhibition zone around each antibiotic disc was measured to assess the susceptibility of each strain. All experiments were conducted in triplicate. Further research was carried out to assess the antifungal activity of the isolated bacterial strains: Lactobacillus plantarum №1, Pediococcus pentosaceus №1, Lactobacillus fermentum. These were tested against the following phytopathogenic micromycetes: Alternaria alternate, Verticillium dahlia, Fusarium spp., Aspergillus flavus, Aspergillus oryzae. The antagonistic properties of the bacterial strains were evaluated using the agar block method (Table 1).
|
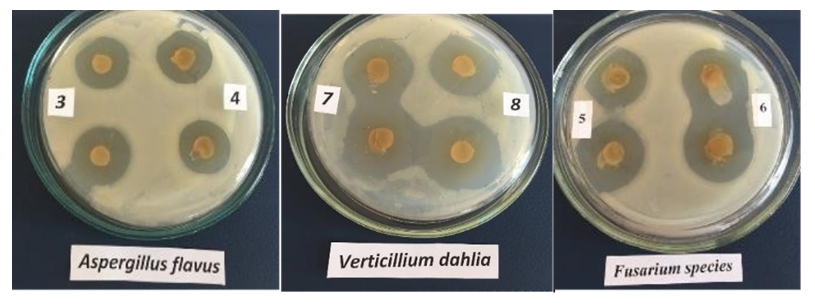 | Figure 2. Antagonistic Properties of Lactic Acid Bacteria |
4. Conclusions
- The antifungal activity of lactic acid bacteria strains was determined by the growth radius of the phytopathogens and it was found that they form a ring from 15 to 35 mm.The isolated strains Lactobacillus plantarum №1, L. brevis №1, L. fermentum №1, and Pediococcus pentosaceus №1 exhibited high antibacterial and antifungal activity against opportunistic and pathogenic microorganisms.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML