-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Virology and Molecular Biology
p-ISSN: 2163-2219 e-ISSN: 2163-2227
2025; 14(6): 99-102
doi:10.5923/j.ijvmb.20251406.02
Received: Aug. 24, 2025; Accepted: Sep. 13, 2025; Published: Sep. 20, 2025

Selection of Salt–Tolerant Safflower Genotypes Using Antioxidant Enzymes
Sarvar Mamatkarimovich Abdurakhmonov1, Tojidin Khamdamovich Kuliev2, Utkir Tulkin ogli Jumanov3, Musulmon Inomjonovich Madrakhimov4, Bakhrom Uktam ogli Almamatov3, Khilola Valijon kizi Zulkhaydarova5
1PhD Student, Gulistan State University, Gulistan, Uzbekistan
2Doctor of Philosophy (PhD) in Agricultural Sciences, Associate Professor, Gulistan State University, Gulistan, Uzbekistan
3Doctor of Philosophy (PhD) in Biological Sciences, Gulistan State University, Gulistan, Uzbekistan
4Independent Researcher, Gulistan State University, Gulistan, Uzbekistan
5Student, Gulistan State University, Gulistan, Uzbekistan
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This article presents data on the influence of soil salinity on the activity of antioxidant enzymes such as superoxide dismutase (SOD), catalase, and malondialdehyde (MDA). The study was conducted on safflower (Carthamus tinctorius L.) genotypes Milyutin–114 and K–435 (introduced from Pakistan). It was found that increasing soil salinity led to a 0.12% and 60.0% increase in SOD activity for Milyutin–114 and K–435, respectively; catalase activity increased by 12.4% and 53.8%; and MDA content increased by 27.9% only in K–435. Based on this data, the K–435 genotype was recognized as salt–tolerant plants.
Keywords: Soil salinity, Antioxidant enzymes, Superoxide dismutase (SOD), Catalase, Malondialdehyde (MDA), Safflower (Carthamus tinctorius L.), Milyutin–114, K–435, Salt–tolerant, Reactive oxygen species (ROS)
Cite this paper: Sarvar Mamatkarimovich Abdurakhmonov, Tojidin Khamdamovich Kuliev, Utkir Tulkin ogli Jumanov, Musulmon Inomjonovich Madrakhimov, Bakhrom Uktam ogli Almamatov, Khilola Valijon kizi Zulkhaydarova, Selection of Salt–Tolerant Safflower Genotypes Using Antioxidant Enzymes, International Journal of Virology and Molecular Biology, Vol. 14 No. 6, 2025, pp. 99-102. doi: 10.5923/j.ijvmb.20251406.02.
1. Introduction
- The salinization of agricultural lands and the search for efficient usage of these lands is one of the most urgent problems today. The relevance of this issue is determined by the reduced ability to obtain high-quality yields from saline fields, while its complexity is explained by the interaction of biotic (biological traits of plants) and abiotic (type and level of soil salinity, climate conditions) factors. Soil salinity acts as a stress factor on plants, leading to the formation of reactive oxygen species (ROS), which negatively affect plant growth, development, and productivity, even causing plant death [1-5].Therefore, the selection and purposeful use of salt–tolerant plants is a priority issue. In this context, safflower is important due to its tolerance to drought and saline conditions. Safflower belongs to the Asteraceae family and the Carthamus genus, and cultivated varieties are derived from Carthamus tinctorius L. Mainly, safflower is used for oil extraction. According to scientific sources, safflower oil is rich in linoleic acid, which helps maintain vascular elasticity. Its flowers contain red and yellow pigments and have medicinal properties. The oil is also used for margarine production. Globally, countries such as Kazakhstan, Russia, Mexico, the USA, and India are considered leaders in safflower cultivation [6].In recent years, evaluating plant tolerance to salinity using antioxidant enzyme activity has gained importance. For example, when bean plants were germinated in 50 and 100 mM NaCl solutions, catalase activity was found to be higher in salt–tolerant genotypes (GS57) than in sensitive ones [7]. Similar studies on sugar beet showed higher ascorbate peroxidase activity in salt–tolerant genotypes [8,9]. An increase in salt concentration in solutions activates ascorbate peroxidase and catalase enzymes [10,11], and this activation helps detoxify hydrogen peroxide, contributing to plant adaptation to salinity [12].Superoxide dismutase (SOD) plays a vital role in protecting organisms against stress factors. It converts superoxide radicals into hydrogen peroxide and molecular oxygen [13]. In soybean under drought stress, increased lipid peroxidation and elevated levels of SOD, peroxidase, catalase, and proline were observed [14]. Catalase functions as a catalyst in breaking down hydrogen peroxide into water and oxygen [15,16], and it plays a role in photosynthesis, cardiovascular tissues, and seed metabolism [17,18]. Increased catalase activity under low temperatures has been recorded in potato and barley [19]. With 200 mM NaCl salt treatment, accumulation of H₂O₂/O₂ was noted in plant leaves [20]. When two pea (Pisum sativum L.) cultivars differing in salt tolerance were irrigated with 70 mM NaCl solution for 15 days, the salt–tolerant (cv. Granada) showed increased APX, GR, and MDHAR activity, whereas these levels remained unchanged in the sensitive cv. Challis [21]. Thus, antioxidant enzyme activity is a significant indicator in evaluating plant resistance to abiotic factors [22].Based on the above literature, it is evident that antioxidant activity increases in stress–resistant plants under stress conditions. Hence, antioxidant enzyme activity is a reliable criterion for selecting stress–tolerant genotypes. As mentioned, safflower is drought– and salt–tolerant, thus is usually cultivated on non–irrigated lands. Recent studies identified promising new genotypes for irrigated conditions such as Nari NH–H–5 and Nari NH–H–15. Research is currently ongoing to select autumn types of safflower [23]. However, there is a lack of studies evaluating antioxidant enzyme activity in safflower under saline soil conditions. Therefore, this study aimed to assess the effect of soil salinity on antioxidant enzyme activity.
2. Materials and Methods
- As the object of investigation, two accessions of Carthamus tinctorius L. (safflower) were selected: the cultivar “Milyutin–114” and the introduced accession “K–435” from Pakistan. These accessions were studied under field conditions with varying degrees of soil salinity.Among the antioxidant enzymes, superoxide dismutase (SOD) activity was measured based on the enzyme’s ability to inhibit the photochemical reduction of nitroblue tetrazolium (NBT), as described in the method [24]. The reaction mixture consisted of 50 mM potassium–sodium phosphate buffer (pH 7.8), 172 µM NBT, 210 µM methionine, 24 µM riboflavin, and 0.1% Triton X–100. The extracts were incubated under light for 10 minutes, and the absorbance was recorded at 535 nm. SOD activity was expressed in arbitrary units (U) per mg of protein per 10 minutes.Catalase (CAT) activity was determined according to the method of Sinha [25], with modifications proposed by Mahmoud H.H. [26]. This method is based on the reduction of hydrogen peroxide by dichromate to chromic acetate in the presence of acetic acid. Fresh plant tissue (500 mg) was ground with liquid nitrogen and extracted in 5 mL of 50 mM phosphate buffer (pH 7.8). The homogenate was centrifuged at 12,000×g for 15 minutes. The reaction was initiated by adding 1000 µL of 0.2 M H₂O₂ to 200 µL of the enzyme extract and was stopped after 1 minute by adding a dichromate–acetic acid mixture. The amount of chromic acetate formed was measured spectrophotometrically at 570 nm. Catalase activity was expressed as µmol H₂O₂ decomposed per minute per mg of protein (µmol H₂O₂ min⁻¹ mg⁻¹ protein).The malondialdehyde (MDA) content, as an indicator of lipid peroxidation, was measured using the thiobarbituric acid (TBA) assay, according to Gur et al. [27]. To 0.5 mL of homogenate, 0.2 mL of 1 mM FeSO₄ and 0.3 mL of 1% Triton X–100 were added, followed by incubation in a water bath at 40°C for 30 minutes with constant shaking. The reaction was stopped by adding 0.2 mL of 3 mM dihydroquercetin. Subsequently, 0.5 mL of 1% Triton X–100, 0.2 mL of 0.6 M HCl, and 0.8 mL of 0.06 M TBA working solution were added. The mixture was heated in a boiling water bath for 10 minutes and then cooled to 15°C for 30 minutes. To stabilize the color, 0.2 mL of 5 mM EDTA and 5–10 mL of ethanol: chloroform mixture (7:3, v/v) were added. The absorbance was measured at 532 nm, with correction for non–specific absorbance at 600 nm. MDA content was expressed as nmol per gram of fresh weight (nmol g⁻¹ FW).
3. Results and Discussions
- According to scientific literature, the activity of superoxide dismutase (SOD) increases under unfavorable climatic conditions such as cold, heat, soil salinity, and drought. This observation was previously noted in the literature review. Most importantly, it has been confirmed that SOD activity increases in stress-tolerant genotypes, making it a crucial criterion in selecting plants resistant to stress environments. Superoxide dismutase, as an antioxidant enzyme, works alongside catalase to protect organisms from reactive oxygen species.For instance, when studying safflower genotypes–salt–tolerant cv. M–CC–190 and salt–sensitive cv. IL–111–in different sodium chloride solutions, the amount of antioxidants increased under saline conditions in the salt–tolerant genotype [26]. Similar findings have been reported by other researchers as well [18-21]. These data indicate that antioxidant enzyme activity is elevated in salt–tolerant genotypes under saline conditions.The same trend was observed in our research. Under mildly saline soil conditions, the amount of superoxide dismutase in the Milyutin–114 variety was 3.207, while in the K–435 accession, it was 2.803. These indicates that under mildly saline conditions, the SOD content in the K–435 accession was lower than in the control. However, when these genotypes were studied under higher soil salinity levels, the SOD content in Milyutin–114 was 3.203, whereas in K–435 it reached 4.503 (see Figure 1).
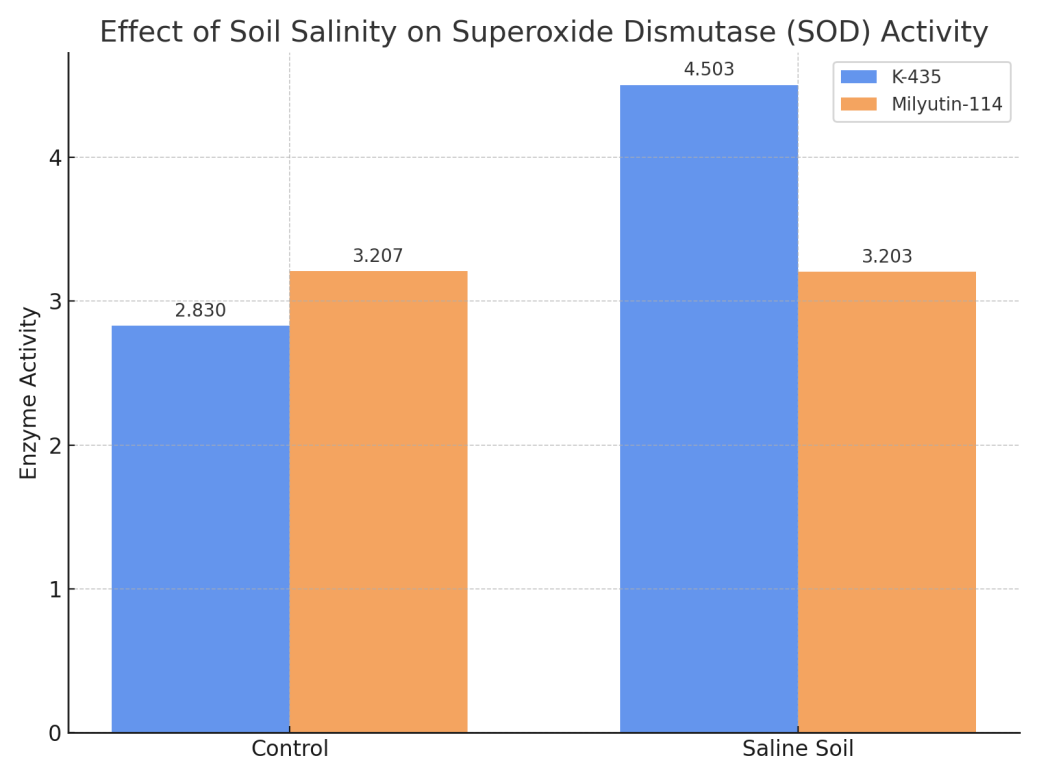 | Figure 1. Effect of soil salinity level on superoxide dismutase (SOD) enzyme activity |
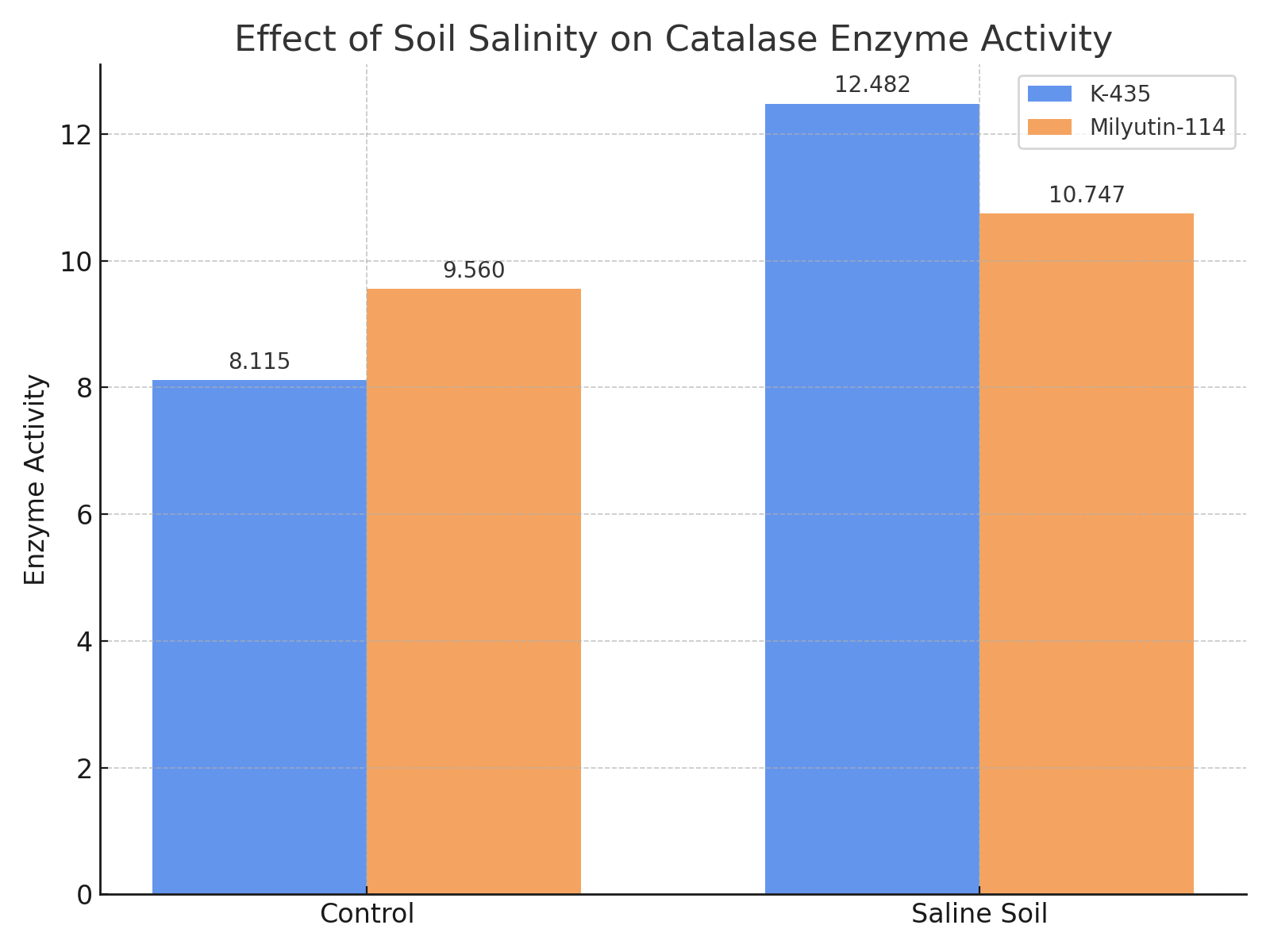 | Figure 2. Effect of soil salinity level on catalase enzyme activity |
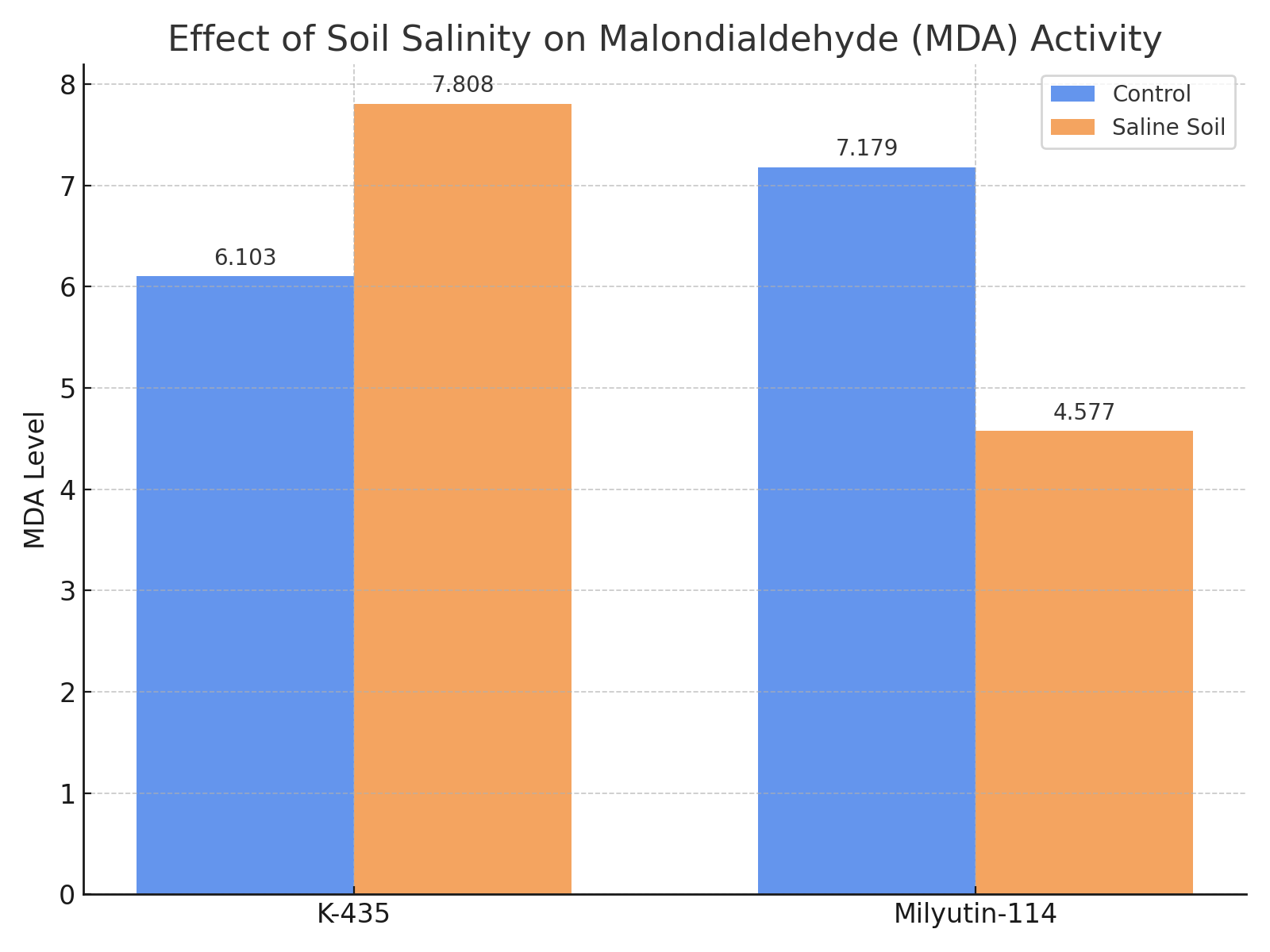 | Figure 3. Effect of soil salinity level on malondialdehyde (MDA) activity |
4. Conclusions
- Based on the obtained results, the following conclusions were drawn:1. An increase in soil salinity led to enhanced activity of antioxidant enzymes. The amount of superoxide dismutase (SOD) ranged from 2.830 to 3.203 under mildly saline conditions, and increased to 3.203–4.503 with higher salinity levels. In this case, SOD content increased by 0.12% in the Milyutin-114 variety and by 60.0% in the K–435 accession;2. Catalase content ranged from 8.115 to 9.551 under mildly saline conditions and increased to 10.747–12.481 with rising soil salinity. Catalase activity increased by 12.4% in Milyutin–114 and by 53.8% in K–435;3. Malondialdehyde (MDA) levels varied by genotype and soil salinity. Under mildly saline conditions, MDA ranged from 6.103 to 7.179, while under more saline conditions, it ranged from 4.517 to 7.808. In the K–435 accession, MDA content increased by 27.9% compared to the control;4. Increased soil salinity enhanced the activity of antioxidant enzymes (superoxide dismutase, catalase, and malondialdehyde) in the K–435 accession compared to the Milyutin–114 variety, supporting the classification of K–435 as salt–tolerant.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML