-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Virology and Molecular Biology
p-ISSN: 2163-2219 e-ISSN: 2163-2227
2025; 14(5): 77-85
doi:10.5923/j.ijvmb.20251405.04
Received: Jun. 22, 2025; Accepted: Jul. 21, 2025; Published: Jul. 31, 2025

Postnatal Cardiac Structural Alterations in Offspring of Mothers Exposed to Experimental Hypothyroidism
Mirzamukhamedov Odiljon Xadjiakbarovich1, Kholieyeva Nigora Udayberdiyevna2, Sayfiddin Khoji Kadriddin Shuhrat ugli3
1Associate Professor of the Pathological Anatomy Department, PhD, Tashkent State Medical University, Tashkent, Uzbekistan
2Assistent of the Pathological Anatomy Department, Tashkent State Medical University, Tashkent, Uzbekistan
3Master’s Degree in “Pathological Anatomy” at the Tashkent State Medical University, Tashkent, Uzbekistan
Correspondence to: Mirzamukhamedov Odiljon Xadjiakbarovich, Associate Professor of the Pathological Anatomy Department, PhD, Tashkent State Medical University, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This study elucidates the pathomorphological alterations in the myocardial tissue of progeny born to female rats subjected to experimentally induced maternal hypothyroidism during the lactational period. The primary objective was to delineate the structural and ultrastructural modifications within the cardiac walls of the offspring under the influence of maternal thyroid hormone deficiency. The experimental model comprised histological and ultrastructural analyses of myocardial specimens from 50 rat pups at distinct stages of early postnatal ontogeny. Results. The offspring of hypothyroid rats caused by experimental methods exhibited pronounced degenerative-dystrophic alterations in myocardial architecture. These alterations were most conspicuous by postnatal day 14 and were histologically characterized by marked perivascular and interstitial lymphohistiocytic infiltration. At the ultrastructural level, significant disorganization of the contractile apparatus, including fragmentation of myofibrils, mitochondrial swelling and cristae disruption, as well as endothelial and pericyte damage in microvasculature, were observed. These changes contributed to the development of intercellular edema and compromise of the vascular barrier integrity, suggesting impaired cardiomyocyte homeostasis and microcirculatory dysfunction as a consequence of maternal hypothyroidism.
Keywords: Heart, Myocardium, Hypothyroidism, Myocytolysis, Mexidol
Cite this paper: Mirzamukhamedov Odiljon Xadjiakbarovich, Kholieyeva Nigora Udayberdiyevna, Sayfiddin Khoji Kadriddin Shuhrat ugli, Postnatal Cardiac Structural Alterations in Offspring of Mothers Exposed to Experimental Hypothyroidism, International Journal of Virology and Molecular Biology, Vol. 14 No. 5, 2025, pp. 77-85. doi: 10.5923/j.ijvmb.20251405.04.
Article Outline
1. Introduction
- Hypothyroidism is one of the most common functional disorders of the thyroid gland, resulting from a prolonged and persistent deficiency of thyroid hormones or a decreased biological response at the cellular level. This condition often remains undiagnosed for extended periods due to its gradual and insidious onset. In mild to moderate hypothyroidism, patients typically feel relatively well, and symptoms are often nonspecific or misinterpreted as fatigue, depression, or pregnancy-related effects, complicating early diagnosis.An elevation in thyroid hormones induces widespread systemic changes. These hormones are critical regulators of cellular energy metabolism, and their deficiency leads to decreased tissue oxygen utilization, reduced energy expenditure, and impaired processing of energy substrates [7,8,12]. In hypothyroidism, the synthesis of energy-dependent enzymes necessary for normal cellular function is disrupted. In severe and chronic cases, hypothyroidism results in mucinous edema known as myxedema, primarily manifesting in connective tissue. This occurs due to excessive accumulation of glycosaminoglycans, which are highly hydrophilic and retain water within tissues.Some literature data studied not only changes in the heart with endocrine pathologies, but also in the vascular system. In more than 90% of cases, especially in older age groups with cardiovascular pathology, the cause may be the development of diastolic dysfunction, the severity of which is traditionally associated with a decrease in the contractility of the heart [4,16].Both thyrotoxicosis and hypothyroidism frequently cause significant cardiovascular complications, elevating these conditions beyond purely endocrine disorders to critical cardiological issues [6,7,11]. Cardiac pathology associated with thyroid dysfunction often predominates in clinical practice, contributing to patient disability and, in severe cases, increased mortality. Clinical manifestations include a broad spectrum of complex arrhythmias, arterial hypertension, and hormone imbalance-induced cardiomyopathy, often progressing to chronic heart failure [1,2]. However, current research on the pathogenetic mechanisms underlying “thyrotoxic” and “hypothyroid” heart diseases remains limited and occasionally contradictory [3,5].Recent studies confirm that cardiovascular disease remains the leading cause of mortality in developed countries [14,15]. The heart is particularly susceptible to damage from endogenous and exogenous factors, which impair its function and contribute to systemic pathological processes [10,12]. Cardiac injury typically begins with alterations in blood vessels and connective tissue frameworks before progressing to the myocardium. Consequently, pathological changes affect both vascular and muscular cardiac structures, leading to quantitative and qualitative modifications that manifest clinically as impaired cardiac function.Objective of the study: This investigation aimed to examine the structural and morphological alterations in different regions of the heart in neonatal rats born to hypothyroid mothers, and to evaluate the potential protective effects of the antioxidant mexidol in mitigating these changes.
2. Purpose of the Research
- The aim of our work was to identify structural and morphological changes in the walls of various parts of the heart of baby rats born to mothers in a state of hypothyroidism and the use of the antioxidant mexidol.
3. Material and Methods
- The object of the study was the hearts of 50 white outbred rats of the following age groups: 3, 7, 14, 21, 30 days. Animals were divided into 3 groups. The first group of animals consisted of female rats receiving mercazolil at a dose of 5 mg per 100 g of body weight for 30 days, then for the month before pregnancy, a maintenance dose of mercazolil was used at a rate of 2.5 mg per 100 g. After pregnancy and during feeding, rats were injected with mercazolil in a maintenance dose of 2.5 mg per 100 g through a probe. As a solvent for mercazolyl used 1% starch paste. The second group of the experiment consisted of female rats who were injected with mercazolilum on the same days of the experiment as in the first group, and after pregnancy they received mexidol at a dose of 0.5 mg per 100 g of weight together with mercazolilum. The third group included female rats of the mother of the control group, which, after birth, rat pups were daily fed on an empty stomach, depending on the term, in the amount of 1 ml distilled water. After each experimental week, the hormone level from the rat caudate vein was determined.
4. Results and Discussion
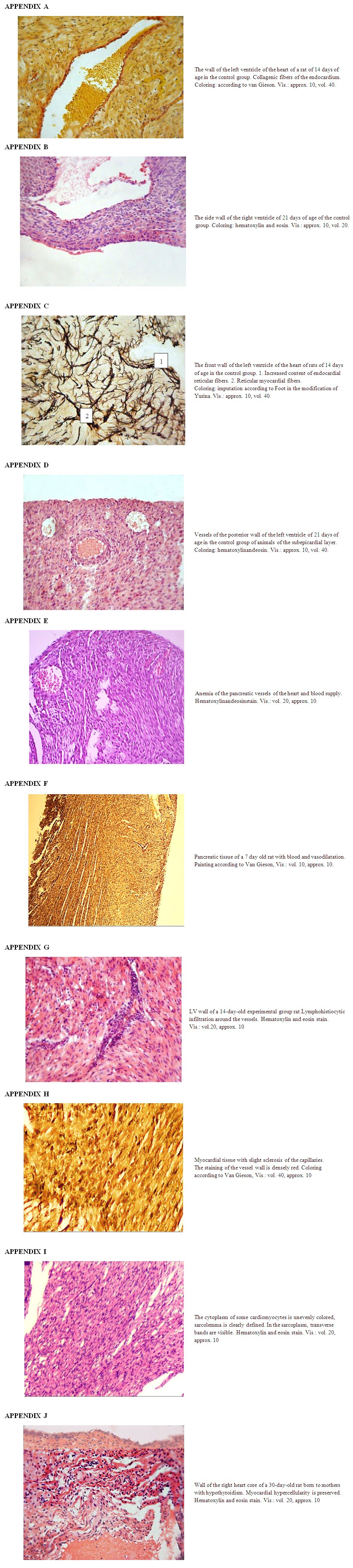
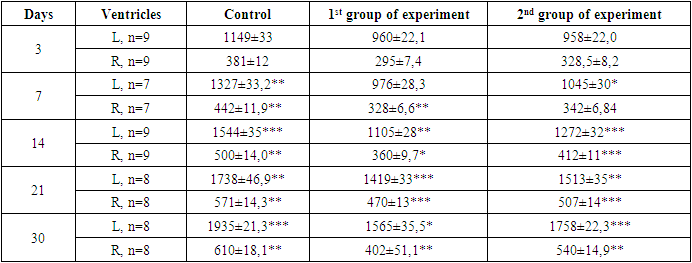
- The control and experimental groups of animals were kept in the same vivarium conditions. At the end of the experiment, the rat pups of the experimental and control groups were killed under ether anesthesia. After that, the heart was isolated from animals, fixed in 10 per cent neutral formalin, followed by piping in alcohols, pouring in paraffin and preparing histological sections. Sections of 8-10 microns thick were prepared from paraffin blocks. Microsections were stained with hematoxylin and eosin, van Gieson.The ventricular endocardium of the control group of animals consisted of longitudinally directed bundles of collagen fibers. A histological section reveals places where longitudinally lying bundles of collagen fibers interwoven with each other. Bundles of collagen fibers located closer to the ventricular myocardium are intertwined with bundles of collagen fibers from connective tissue layers located between bundles of cardiomyocytes of the myocardial inner layer (See App. A). The bundles of elastic fibers of the ventricular endocardium lie loosely compared to bundles of collagen fibers. In bundles of fibers adjacent to the myocardium of the ventricles, the density of arrangement increases, the direction of which changes from longitudinal to oblique, and are interwoven with bundles of connective tissue located between bundles of cardiomyocytes of the inner layer of the myocardium. Reticular fibers in the endocardium of the ventricles are located close to each other.Upon further study of the slice, the cardiomyocytes of the outer layer of the myocardium are directed longitudinally, in the middle layer circular directed beams are detected; the inner layer contains weakly obliquely oriented bundles of cardiomyocytes. The inner bundles of fibers as they approach the endocardium acquire a more oblique direction and pass into the papillary muscles. A study of the direction of the bundles of ventricular myocardial fibers showed that the circularly directed layer does not always have a clear orientation. The bundles of fibers of the middle layer on the lower wall of the ventricles are directed obliquely and deviate towards the endocardium. Between the layers of ventricular myocardial cardiomyocytes, the border is weakly expressed. They fit snugly together. The inner layer of the myocardium consists of parallel located bundles of cardiomyocytes running in parallel with the endocardium. The outer layer of the myocardium has a loose structure in which cardiomyocytes are located in different directions (See App. B).In the middle layer of the myocardium of the left ventricle of the heart, bundles of cardiomyocytes are located perpendicular to the inner layer. In the center of cardiomyocytes, 1-2 oval-shaped nuclei are determined. The nucleus of cardiomyocytes is located in the center of the cell, while myofibrils are located on the periphery. In the right ventricle, bundles of cardiomyocytes in the layers of the myocardium are similar to the left ventricular myocardium. But unlike the left ventricle in the myocardium of the right ventricle, the thickness of the circular layer of cardiomyocytes is 2-3 times thinner than the thickness of the longitudinal layers of cardiomyocytes.In the myocardium of the ventricles of the heart, depending on the site, bundles of collagen fibers have different directions. At the top of the heart, the bundles of collagen fibers are directed obliquely, while part of the bundles of collagen fibers changes direction from oblique to longitudinal. In the inner layer of the myocardium, bundles of collagen fibers lie in the longitudinal direction, separating bundles of cardiomyocytes from each other. In the middle layer of the myocardium, collagen fibers form bundles having a circular direction. When studying the outer layer of the myocardium, bundles of collagen fibers lie obliquely between bundles of cardiomyocytes. In the inner layer, the reticular fibers lie longitudinally and in the region of the apex of the heart they are intertwined with the reticular fibers from the outer layer of the myocardium of the heart. In the middle layer of the myocardium, the reticular fibers are located in a circular direction between the bundles of cardiomyocytes. Reticular fibers around bundles of cardiomyocytes form a network of various sizes and shapes (See App. C). The direction of the bundles of connective fibers depends on the direction of the cardiomyocytes.The interventricular septum consists of two longitudinal and one circular layer. The longitudinal layers on the left and on the right are from the corresponding longitudinal layers of both ventricles, and the middle circular layer is formed due to the circular layer of the left ventricle of the heart. The internal diameter of the arteriole ranges from 9.5 to 15.2 microns and an average of 11.7 ± 0.6 microns. The inner diameter of the venules ranges from 15.7 to 20.5 microns. Myocardial sinusoids are elongated, oval or irregular in shape. The thickness of the inner diameter of the capillaries is from 5.7 to 11.4 microns, an average of 9.3 ± 0.6. Myocardial blood vessels are direct along the bundles of cardiomyocytes (See App. D). Around the cardiomyocytes and blood vessels are bundles of collagen and elastic fibers. The fibrous structure of the connective tissue of cardiomyocytes connecting with the blood vessels captures the capillaries around the muscle fibers.In the epicardium of the ventricles of the heart, bundles of collagen and elastic fibers lie longitudinally, and have a higher density than bundles of collagen and elastic fibers of the endocardium. In the epicardium of the ventricles, the reticular fibers are located longitudinally.The histological picture of 3-day-old rats undergoing hypothyroidism did not reveal significant differences compared with the control group. Cardiomyocytes had an oblong shape, formed muscle fibers, in the center of the fiber an oval-shaped nucleus was determined and myofibrils were clearly differentiated. In the subepicardial zone of the myocardium, dilated and full-blooded veins were detected with signs of redistribution of blood by the presence of clotted red blood cells, and in the myocardial stroma, beginning edema was observed. The endocardium is also somewhat thickened and uneven, in some places foci of depression and deepening in the form of cracks and vessels of Tebesia are visible. Endothelial cells are somewhat hypertrophied and hyperchromic, sometimes with foci of enlightenment in the basement membrane.A morphological study of the heart of 7-day-old pups born to mothers in a state of hypothyroidism, with minor changes in the form of expansion of visible vessels (See App. E). An increase in the permeability of microvessels and vessels of the venous link was accompanied by the release of the liquid part of the blood through the vessel wall to the surrounding connective tissue (See App. F). Due to inflammatory infiltrate, the vascular wall thickens and the lumen narrows. These discirculatory disorders lead to perivascular edema, loosening of the intramuscular space. At the same time, cardiomyocytes are loosened with the development of dystrophy and vacuolization in their cytoplasm. Myofibrils have a granular appearance, of different thickness and density. During this period, the epicardium of the heart is significantly thickened due to the proliferation of young connective tissue cells and swelling of fibrous structures. The endocardium is also swollen, in places forms uneven thickenings from proliferative endothelial cells.At the age of 14 days, destructive and inflammatory phenomena join the above-described discirculatory and dystrophic changes. In the myocardial stroma, increased swelling was noted mainly in the perivenular and pericapillary spaces. Collagen fibers are swollen, loosened, in some places there is a separation of collagen bundles, swelling of the main substance of the connective tissue with the initial signs of surface disorganization. Connective tissue cells are also swollen, their nuclei slightly increase in size. Vascular disorders are common, endothelial cells in the vessels, acquire a rounded shape. In the cytoplasm of cardiomyocytes, small vacuoles were found filled with a clear cytoplasmic fluid, i.e. hydropic dystrophy develops. Intracellular edema is focal in nature, along with dystrophically altered cardiomyocytes, unaffected cells are found. In the myocardium of the left ventricle of the heart, around the vessels and in places in the stroma, inflammatory foci consisting of lymphohistiocytic cells appear (See App. G). In this case, cardiomyocytes are loosened with the development of dystrophy and vacuolization in their cytoplasm. Myofibrils have a granular appearance, different thickness and density. The endocardium is also swollen, in places forms an uneven thickening of proliferative endothelial cells. At this time, the epicardium of the heart is significantly thickened due to the proliferation of young connective tissue cells and swelling of fibrous structures. A histochemical study reveals a significant decrease in the number of collagen structures in the composition of the epicardium, instead of them, the content of SHIK-positive substance increases, especially in the walls of blood vessels. Endothelial cells are somewhat hypertrophied, hyperchromic, sometimes with foci of enlightenment in the basement membrane.The study process of growth dynamics of the wall thickness of both the left and right ventricles of the heart of rat pups born by mothers in hypothyroidism state, depending on different parts of the heart, showed that the wall thickness of all departments is less than the by control indices. Comparing the thicknesses of wall of the right and left ventricles of the animals of the experimental group with the control group, a significant lag in myocardial indices is revealed. These changes are being most pronounced in pups of 14 days of age. The thickness of the left ventricular myocardium is less than that of the control group by 29 per cent. The thickness of the right ventricle compared with the control group is less by 17 per cent (Table).On the 21st day, vascular disorders persist in the myocardium: pronounced plethora of veins, stasis, numerous perivascular hemorrhages of a diapedetic nature and an increase in the intensity of edema is noted. Edematous fluid is located between the muscle fibers, as if pushing them apart. Swelling of not only connective tissue cells, but also their processes was revealed. The swollen processes of neighboring fibroblasts are in contact with each other and, as it were, form syncytium. In the myocardial stroma, numerous small infiltrates from lymphocytes, histiocytes and fibroblasts. In cardiomyocytes, signs of protein hydropic dystrophy with the development of intracellular edema are observed, numerous foci of plasmolysis throughout the myocardium are determined. Plots of plasmolysis (intracellular myocytolysis) look like optically empty spaces. With intracellular myocytolysis, dissolution of myofibrils in certain sections of the fiber along its length is noted. In adjacent areas, myofibrils are preserved. With silvering according to Foote, the formation of reticular fibers in the form of coarse clumps and intermittent thick dark brown fibrous structures is determined. A histochemical study using the Van Gieson method reveals loosening and a decrease in the number of collagen structures, especially in the epicardium, and an increase in the content of SHIK-positive substance. Elastic fibers of the endocardium and in the intramuscular stroma in a state of loosening and tearing due to edema and disorganization of the connective tissue.The study process of the dynamics of growth of wall thickness of both the left and right ventricles of the heart of rat pups born by mothers in a state of hypothyroidism with the use of mexidol depending on different parts of the heart showed that the wall thickness of all departments is less than by the control indices. Comparing the wall thicknesses of the right and left ventricles of the animals of the experimental group with the control, a significant lag in myocardial indices is revealed. These changes are being most pronounced in pups of 21 days of age. The thickness of the left ventricular myocardium is less than that of the control group by 29 per cent. The thickness of the right ventricle compared with the control group is less by 17 per cent (Table).After 30 days, interstitial edema intensifies and spreads to the entire myocardium, reaching the greatest value in the perivenular spaces. Due to edema, swelling of collagen fibers, their delamination and shedding are observed. The main substance swells and collapses, signs of disorganization of the connective tissue appear (See App. H). When stained with toluidine blue, the phenomenon of metachromasia is observed. Dystrophic changes in the myocardium acquire a diffuse character, increased resorption of the cytoplasm, intracellular edema is noted. The foci of plasmolysis are numerous and larger than on the 21st day. Intracellular myocytolysis extends to the entire myocardium; total damage to cardiomyocytes is noted. In the focus of myocytolysis, most myofibrils are absent, single fibrils are visible. At the same time, marked loosening of the subendocardial and intramural layers of the heart wall due to edema and dissociation of cardiomyocytes is noted.The study process of the growth dynamics of thickness of wall of both left and right ventricles of a heart of baby of intact rats at different stages of postnatal ontogenesis depending on the parts of the heart walls has showed that by the 30th day of the current study, comparing to 3 days of age, thickening occurs on average in all parts of the heart 1.7 times, only in the interventricular septum - 1.5 times. These morphometric data are confirmed by microscopic rearrangements occurring in the dynamics of postnatal ontogenesis (Table).The subepicardial layer of the myocardium remains dense, where cardiomyocytes form large muscle bundles. Inflammatory infiltrate and sclerosis are defined around the vessels. When staining according to the Weigert method, it is noted that in the walls of the arteries the inner elastic membrane is thickened, winding, sometimes homogenized. In the muscle layer and in the surrounding connective tissue, elastic fibers are fibrous and lysed.In cardiomyocytes with small foci of myocytolysis, the nuclei are preserved. In cardiomyocytes with large foci of myocytolysis, the nuclei are destroyed with the development of collicational necrosis. Remains of fibers in the form of tubules surrounded by a sarcolemma are found in foci of collication necrosis; the contents are not stained. Sarcolemma is thickened and well stained with picrofuxin. The described tubules are collapsed, around them there are excessive deposits of glucosaminglycans.In some areas, counter-abnormalities and small foci of coagulation are determined, fragmentation of muscle fibers is observed in some places. However, collision processes dominate in the myocardium.Since metabolic imbalance primarily affects the energy supply system of cells, the therapy should be aimed at increasing energy generation and increasing myocardial resistance to hypoxia. The second experimental group included 26 rats, which were daily injected with mercazolyl and antioxidant a-tocopherol.In rat pups 3 days old born from mothers to hypothyroidism and receiving mexidol, the normal histological picture is determined in the rat myocardium, pathohistological changes in the myocardium are not detected.After 7 days of birth, vascular disorders in the form of plethora and expansion of veins, stasis, plasmorrhagia are noted (See App. E). Vascular disorders persist, there is an increase in plasmorrhagia, plasma impregnation and fibrinoid swelling of the walls of the arteries.After 14 days from the start of the experiment, against the background of the use of tocopherol in the myocardial stroma, a small edema was found mainly around the veins. In individual cardiomyocytes, intracellular edema was detected in the form of accumulations in the cytoplasm of vacuoles filled with tissue fluid.Intracellular edema is focal in nature. In the myocardial stroma, vascular disorders persist in the form of plethora, stasis and venous stasis. When staining with toluidine blue, metachromasia was not detected; fibrinoid swelling of the walls of the artery is observed.After 21 days, edema was found in some places in the myocardial stroma, which is focal in nature and has a lower intensity than in the first experimental group. In areas of edema, collagen fibers swell and homogenize. There is a superficial disorganization of connective tissue with the accumulation of glycosaminoglycans. On histological preparations, mucoid swelling is observed (See App. I).In places in the cytoplasm of cardiomyocytes, hydropic dystrophy is observed, which is focal in nature. The transverse striation of muscle fibers is preserved, in some area’s myofibrils swell. Vascular disorders become less intense.After 30 days, against the background of daily use of the antioxidant tocopherol, partial relief of vascular disorders is noted. In the myocardial stroma, edema develops, which extends to almost the entire myocardium, however, the intensity of the edema is less pronounced compared with the experimental group I. In the connective tissue of the myocardial stroma, swelling and decomposition of the main substance, accumulation of glucosaminglycans, swelling, homogenization and partial decomposition of collagen fibers with the formation of a fibrinoid are observed. At this time, for the first time in the stroma, small focal infiltrates from lymphocytes, histiocytes, and fibroblasts were detected (See App. J).At the indicated times, a picture of intracellular edema is observed in the myocardium and for the first time against the background of tocopherol use, small foci of plasmolysis (myocytolysis) appear. These foci are optically empty portions of the cytoplasm with complete preservation of the plasmolemma. Compared with the experimental group I, the processes of myocytolysis are focal in nature.Thus, with the daily use of the antioxidant a-tocopherol in rats of the experimental group II, no histological changes in the myocardium were detected in the first 7 days. The first signs of myocardial damage (beginning periventricular edema of single cardiomyocytes) appear on the 14th day, that is, 7 days later than in the first experimental group. An expanded picture of a change in the myocardium such as diffuse intracellular edema, myocytolysis was detected on the 28th day, then the network was later compared by comparison in the first experimental group. Destructive processes with total damage to the myocardial fibers in the experimental group II of rats were not detected. Regenerative processes with the replacement of damaged myocardial cells with connective tissue proceeded more intensively in comparison with the experimental group I. The above suggests that a-tocopherol has a protective effect on the myocardium under conditions of hypothyroidism.
5. Findings
- 1. In transient experimental hypothyroidism, the use of mercazolil in animals in the ventricular myocardium results in dystrophic, destructive and atrophic changes in cardiomyocytes, diffuse edema and stromal fibrosis. The first signs of hypothyroidism were detected on days 7-14, a detailed picture develops on the 21st day.2. When using an antioxidant in the myocardium of experimental animals, signs of hypothyroidism occur on the 21st day, and a detailed picture on the 30th day.3. The intensity and prevalence of morphological changes are less pronounced, destructive changes in the myocardium are not detected.The use of antioxidants in experimental hypothyroidism in laboratory animals has a protective effect and prevents the development of severe destructive changes in the myocardium.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML