-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Virology and Molecular Biology
p-ISSN: 2163-2219 e-ISSN: 2163-2227
2025; 14(4): 56-59
doi:10.5923/j.ijvmb.20251404.04
Received: May 12, 2025; Accepted: Jun. 13, 2025; Published: Jun. 21, 2025

Effect of Prune Dwarf Virus (PDV) on the Total Protein Content in Prunus Avium
Amindjonova G. K.1, Fayziyev V. B.2, 3, Bekmatova E. E.2, 3, A. A. Saydullayev4
1PhD Student of Samarkand State Veterinary Medicine University of Animal Husbandry and Biotechnology of the Tashkent Branch, Uzbekistan
2Doctor of Biological Sciences, Professor, Chirchik State Pedagogical University, Chirchik, Uzbekistan
3PhD Student of Chirchik State Pedagogical University, Chirchik, Uzbekistan
4Tashkent Medical Academy Chirchik Branch, Chirchik, Uzbekistan
Correspondence to: Amindjonova G. K., PhD Student of Samarkand State Veterinary Medicine University of Animal Husbandry and Biotechnology of the Tashkent Branch, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Plant viruses seriously affect the development of agricultural crops. Prune dwarf virus (PDV) is one of the viruses that damage plants belonging to the genus Prunus and negatively affects the growth and development of the plant. It also causes the quality of fruits to decrease. This study aimed to evaluate the effect of PDV on the total protein content of Prunus avium. In this study, the effect of Prune dwarf virus (PDV) on total protein content of Prunus avium (cherry) was analyzed. It can be seen that nitrogen is reduced by 20% and total protein by 27.6% in Bahor variety, and nitrogen by 22.7% and total protein by 32.5% in Ziroat variety. The effect of PDV on plant metabolism and physiological processes in the Bahor variety was evaluated through experimental studies. According to the results, a decrease in the amount of total protein was observed in samples infected with PDV.
Keywords: Prune dwarf virus, Prunus avium, Metabolism, Physiological processes, Total protein, Nitrogen, Productivity, Enzymes
Cite this paper: Amindjonova G. K., Fayziyev V. B., Bekmatova E. E., A. A. Saydullayev, Effect of Prune Dwarf Virus (PDV) on the Total Protein Content in Prunus Avium, International Journal of Virology and Molecular Biology, Vol. 14 No. 4, 2025, pp. 56-59. doi: 10.5923/j.ijvmb.20251404.04.
Article Outline
1. Introduction
- Cherries (Prunus avium) are one of the world's valuable stone fruits, distinguished by their richness in minerals, antioxidants and anti-inflammatory properties. Uzbekistan is one of the leading countries in the world in terms of cherry cultivation, and occupies an important place in the economy and agriculture of our country. According to the Statistics Agency, in 2022, Uzbekistan produced 21.8 million tons of fruits and vegetables, which is 3.8% more than in 2021, and 12.9% more than in 2017. Uzbekistan ranks fourth in the world in terms of cherry cultivation. In the first five months of 2023, Uzbekistan exported cherries worth a total of 40 million US dollars to 15 countries, with Russia (19.8 thousand tons), Kazakhstan (6.4 thousand tons) and Kyrgyzstan (6.1 thousand tons) leading the way [3]. Cherries are not only delicious, but they also have health benefits. Cherries contain high levels of antioxidants, which fight free radicals and protect the body from oxidative stress [5]. Studies show that cherries have anti-inflammatory properties, which can help reduce muscle damage. The vitamin C and other nutrients in cherries help strengthen the immune system. Cherry orchards in our country cover an area of more than 30 thousand hectares, and currently more than 10 varieties are grown (black cherry, yellow cherry, Bahar, Jinetreed, Skina, Ziroat, Sarvi-surkhani, Napoleon, Valery Chikalov, etc.) [2].Increasing crop yield and improving fruit quality are important issues in modern agriculture. However, there are various phytopathogens that affect plant productivity, among which viruses play an important role.Prune dwarf virus infects fruit trees of the Prunus genus, including cherry (Prunus avium), plum (Prunus domestica), peach (Prunus persica), and other stone fruits. This virus reduces the rate of growth of plants, reduces the quality and productivity of fruits. PDV belongs to the family of Ilarviruses and has a filamentous structure without a virus shell. This virus is a single-stranded RNA virus with a genome of approximately 8.4 kb that encodes numerous replication, movement, and capsid proteins [6]. PDV is mainly spread in the following ways: Transmission through grafting and other vegetative propagation methods. It can be spread through used garden tools (scissors, knives). It can also be spread through dust by beetles and other pollinating insects, and in some cases, pests can be carriers of the virus [7]. The main symptoms observed in plants infected with PDV are: yellowing and mosaic of leaves, reduced stem growth (stunting), small and deformed fruits, reduced photosynthetic efficiency, and a significant reduction in yield. These symptoms are caused by the effects of PDV on the metabolism of the host plant. PDV infection leads to a decrease in total protein synthesis, impaired antioxidant enzyme activity, and increased physiological stress symptoms [5,6].Virus-infected plants undergo physiological and biochemical changes, which can lead to a decrease or alteration in protein synthesis. The virus affects protein levels through the following mechanisms:decrease in the process of photosynthesis → decrease in carbohydrates and amino acids;changes in metabolism → decrease in the activity of enzymes affecting protein synthesis;stress state in cells → increased protein breakdown;Therefore, the main goal of this study was to study the effect of Prune dwarf virus on physiological processes in Prunus avium plants.
2. Research Area
- Cotton, grain, vegetable, and fruit cultivation are widespread in the Tashkent region. The region supplies other regions of Uzbekistan with agricultural products. The climate of the region is dry subtropical, very hot in summer and mild in winter. The summer temperature in the region can be higher than +35°C in June, July and August. The winter temperature in January varies from -5°C to +5°C. The annual precipitation is around 100-300 mm, mainly due to spring and fall rains [15,16].Healthy and Prune dwarf virus-infected cherry varieties of Bahor and Ziroat, taken from cherry fields in the Chirchik city and Piskent districts of the Tashkent region, were used as research samples. The research work was conducted at the "Molecular Biology and Bioinformatics" scientific research laboratory of the Department of Biology of the Chirchik State Pedagogical University, as well as at the "Low Molecular Biologically Active Modifiers" laboratory of the Institute of Bioorganic Chemistry of the Academy of Sciences of Uzbekistan. For the study, samples were taken from 20 points in the cherry orchard. Four leaves were collected from each point, for a total of 80 plant leaves, including the leaves of the plant. To maintain the quality of the analysis, each sample was placed in separate sterile polyethylene bags, placed in a thermos bag with special ice packs (+4°C), and quickly transported to the laboratory.The level of plant virus infection was visually analyzed based on the collected samples. PDV was detected when infected samples were analyzed using the classical PCR method. Monitoring was conducted to differentiate the virus from other similar phytopathogenic viruses. As part of the study, leaves from cherry varieties - Bahor and Ziroat - were taken and a comparative analysis of virus-infected and healthy samples was conducted.
3. Analysis of Used Methods
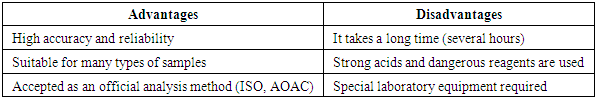
- Various methods are used to determine total protein. The most common methods are: Biuret method – the simplest and most widely used method. Lowry method – based on the Biuret reaction, with the addition of folin-phenol reagent. Bradford method – a method for determining protein levels using Coomassie Brilliant Blue G-250 dye.During the scientific study, the Kjeldahl method was used to determine the total protein content. The Kjeldahl method is a method based on the determination of organic nitrogen (nitrogen in protein). It is used to determine the amount of protein in food and biological materials. The Kjeldahl method is one of the most widely used classical chemical methods for determining the amount of total protein in food, feed, soil, and biological materials. This method is based on determining the nitrogen content of proteins, and allows calculating the protein concentration based on the total nitrogen content [9,10,13].Steps of Keldel's method1. Sample breakdown (digestion).The sample is heated with strong sulfuric acid (H₂SO₄). As a result, organic matter decomposes, producing nitrogen in the form of ammonium sulfate ((NH₄)₂SO₄). In this process, catalysts are added (copper (Cu), selenium (Se) or titanium (Ti)) and the organic matter is decomposed during heating [9,13].2. Neutralization and distillation.After decomposition, sodium hydroxide (NaOH) is added to the ammonium sulfate formed, releasing ammonia (NH₃). The separated ammonia is distilled using steam and collected in a boric acid (H₃BO₃) solution.3. Titration.The amount of NH₃ released is determined by titration with an acid (usually HCl or H₂SO₄). Based on the titration result, the nitrogen content is calculated.4. Calculate the amount of protein.The resulting amount of nitrogen is multiplied by the protein coefficient (usually 6.25) to calculate total protein:
|
 • V1 – the volume of sodium hydroxide solution consumed in the test experiment.• V0 – the volume of sodium hydroxide solution used in the control experiment.• K – concentration of the solution.• m – mass of the analyzed sample.
• V1 – the volume of sodium hydroxide solution consumed in the test experiment.• V0 – the volume of sodium hydroxide solution used in the control experiment.• K – concentration of the solution.• m – mass of the analyzed sample.4. Results and Their Analysis
- According to the results of the study, it was determined that the Bahor variety of cherries was infected with PDV. The total protein content of leaves of Prunus avium was determined using high and low levels of total protein content in damaged and healthy leaves of both varieties using HPLC (Figure 1).
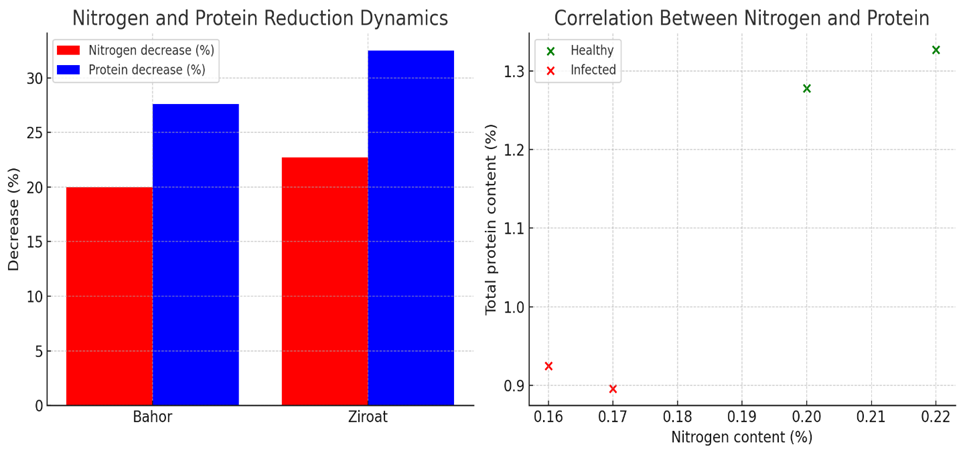 | Figure 1. Dynamics of nitrogen and protein depletion in cherry varieties and the relationship between nitrogen and protein |
5. Conclusions and Suggestions
- The PDV virus reduces protein synthesis and causes the following changes in the cherry plant: Leaf discoloration (chlorosis) - less green pigment is produced. Leaf shrinkage - cells do not divide normally due to impaired protein synthesis. Significant drying and shedding - leaf tissue weakens. Proteins in cherry leaves play an important role in photosynthesis, nutrient distribution, and disease resistance. Reduced protein synthesis due to PDV infection results in leaf weakness and reduced yield. To increase the total protein content in diseased plants, it is necessary to enrich the soil with nitrogen, control diseases, and ensure optimal climatic conditions. In short, PDV has a serious impact on the physiological and metabolic processes of plants, causing a decrease in yield. To effectively combat the virus, it is important to use molecular diagnostic methods, preventive measures, and develop varieties with high immunity. Future research should focus on further understanding the molecular mechanisms of PDV and reducing its pathogenicity.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML