-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Virology and Molecular Biology
p-ISSN: 2163-2219 e-ISSN: 2163-2227
2025; 14(3): 27-30
doi:10.5923/j.ijvmb.20251403.01
Received: May 13, 2025; Accepted: May 28, 2025; Published: Jun. 7, 2025

Evaluation of the Development of Neurodenegenerative Processes in a High-Fat Diet
Mashkhura I. Abdullayeva1, Feruza Kh. Inoyatova2
1Post Graduate Student, Tashkent Medical Academy, Tashkent, Uzbekistan
2Professor, Tashkent Medical Academy, Tashkent, Uzbekistan
Correspondence to: Mashkhura I. Abdullayeva, Post Graduate Student, Tashkent Medical Academy, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This study evaluated changes in the amount of β-amyloid (Aβ) protein and LRP1 protein, which is involved in its clearance, in a modeled nonalcoholic fatty liver disease (NAFLD) based on a high-fat diet. The results showed that Aβ protein levels gradually increase in blood and brain regions in NAFLD, while LRP1 protein levels decrease in liver, blood, and brain tissues. These changes are associated with impaired Aβ clearance and increased risk of neurodegeneration, confirming the complex interplay between liver dysfunction and brain pathology. The results of the study suggest that β-amyloid and LRP1 proteins may be important biomarkers in the development of neurodegenerative processes in NAFLD. This necessitates the development of therapeutic approaches that target the liver to prevent cognitive impairment associated with NAFLD.
Keywords: β-amyloid (Aβ), LRP1 protein, Nonalcoholic fatty liver disease (NAFLD), High-fat diet, Neurodegeneration, Protein clearance
Cite this paper: Mashkhura I. Abdullayeva, Feruza Kh. Inoyatova, Evaluation of the Development of Neurodenegenerative Processes in a High-Fat Diet, International Journal of Virology and Molecular Biology, Vol. 14 No. 3, 2025, pp. 27-30. doi: 10.5923/j.ijvmb.20251403.01.
Article Outline
1. Introduction
- Nonalcoholic fatty liver disease (NAFLD) is currently the most common chronic liver disease. NAFLD affects approximately 25% of the world's population, and this figure is expected to increase to 33.5% by 2030 [1].Nonalcoholic fatty liver disease is increasingly important in the pathogenesis of neurodegeneration and is a risk factor for mild cognitive impairment and dementia.Dementia is a common disease worldwide, with an estimated 50 million people suffering from dementia and this number is expected to increase annually [2]. According to the World Health Organization, the number of people with dementia will reach 82 and 152 million by 2030 and 2050, respectively [3].NAFLD is associated with a decrease in the hepatic expression of proteins involved in the clearance of circulating β-amyloid (Aβ) (LRP1-low-density lipoprotein receptor-related protein, LDLR-low-density lipoprotein receptor) and Aβ catabolism (insulin-degrading enzyme-IDE, neprilysin-NEP). Impaired hepatic Aβ degradation may contribute to the increase in circulating Aβ, which may contribute to the increased accumulation of Aβ in the brain and the development of Alzheimer's disease [4,5]. The involvement of Aβ, LRP1 and ApoE (especially the ApoE4 allele) in the pathogenesis of neurodegeneration and Alzheimer's dementia is very complex and requires further studies.
2. Purpose of the Research
- The purpose of this study was to investigate the relationship between nonalcoholic fatty liver disease (NAFLD)—induced by a high-fat diet—and the accumulation of β-amyloid (Aβ) protein alongside the reduction of LRP1 protein, a key mediator in Aβ clearance. Specifically, the research aimed to evaluate changes in Aβ and LRP1 levels in the blood, liver, and brain tissues in a NAFLD model.
3. Materials and Methods
- 8-10-week-old male Wistar rats were used as subjects. The experimental fatty liver hepatosis model was induced by daily supplementation of melted beef fat to the animal diet based on a high-fat combined diet and by giving 10% fructose and 10% glucose solution instead of water.On the appropriate days of the study, the rats were decapitated in a cold room at a temperature of 0°-+2°C and the blood of the animals was collected. Then, the collected blood was left at +4°C for 30 minutes, centrifuged at 3000 rpm, and the serum was collected. The brain and liver were isolated in the cold, washed in Tris-HCl buffer, pH 7.4, and frozen in liquid nitrogen. The amount of β-amyloid in the serum, cerebral cortex, and hippocampus, and the amount of LRP1 in the serum, liver, cerebral cortex, and hippocampus were determined.
4. Results and Discussion
- Neurodegenerative processes are characterized by the accumulation of amyloid as a result of impaired clearance of pathological β-amyloid protein. Therefore, β-amyloid protein is a very important indicator in the diagnosis of neurodegenerative diseases. For this reason, we also determined the amount of β-amyloid protein, which indicates the development of neurodegenerative processes. The results of our studies showed an increase in the levels of this protein in the serum, hippocampus and cortex of the brain in fatty hepatosis (Figure 1). In particular, at 12, 16 and 20 weeks of our study, the levels of β-amyloid protein in the serum increased by 20.7%; 30.33% and 48.81% compared to the intact group; in the hippocampus it increased by 4.81%; 9.86% and 17.6%. In the area of the cerebral cortex, β-amyloid protein values are 5.14%, respectively; It was found to increase by 10.59% and 12.8%.
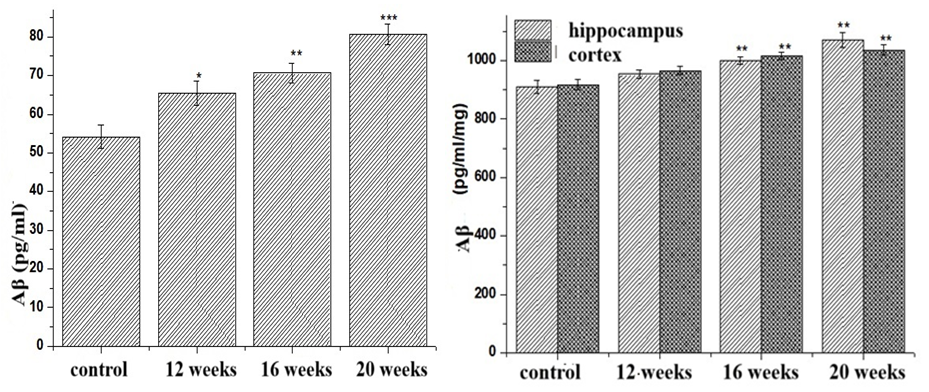 | Figure 1. Dynamics of Aβ in experimental NAFLD |
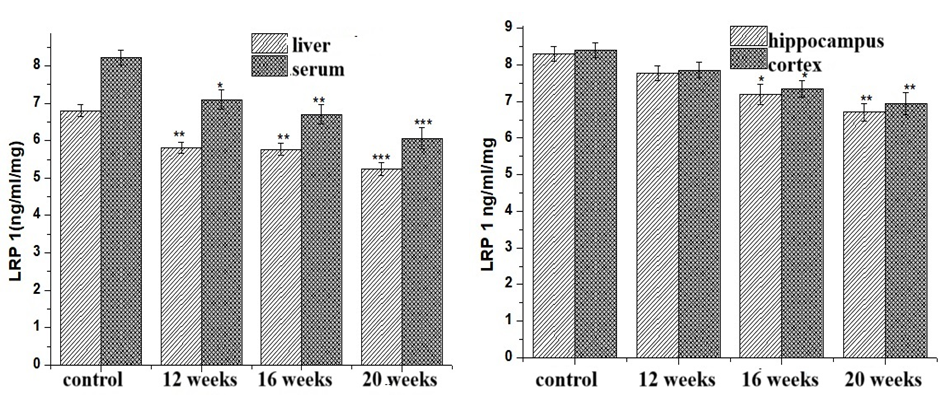 | Figure 2. Dynamics of LRP 1 protein in experimental NAFLD |
5. Conclusions
- In fatty liver disease, an increase in the amount of β-amyloid protein in the serum and brain, and a decrease in the amount of LRP1 protein, which clears them, were found in the liver and blood. The obtained results indicate the development of neurodegenerative processes in a high-fat diet.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML