-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Virology and Molecular Biology
p-ISSN: 2163-2219 e-ISSN: 2163-2227
2024; 13(2): 32-35
doi:10.5923/j.ijvmb.20241302.03
Received: Jun. 2, 2024; Accepted: Jun. 17, 2024; Published: Jun. 21, 2024

Determination of Bitter Melon (Momordica Charantia L.) Genes Resistant to Different Stress Factors
Turgunov Jalaluddin Rahmonali Son 1, Naraliyeva Nasibakhan Mamanovna 2
1Andijan Institute of Agriculture and Agrotechnologies, Uzbekistan
2Doctor of Biological Sciences, Andijan State University, Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

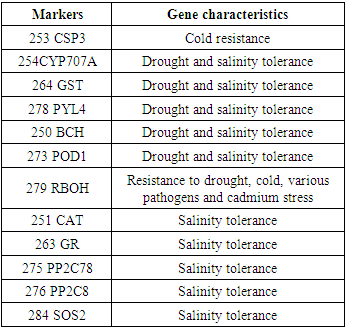
In recent years, studies have been conducted on the effects of drought on specific characteristics of Momordica charantia L. gene expression and metabolic changes in Momordica charantia L. seedlings in response to low temperature stress. However, despite the fact that abiotic stresses such as drought, temperature extremes, salinity, and heavy metals can reduce plant growth and productivity by more than 50%, the presence of tolerance genes in the genome of M. charantia not enough studies have been conducted. For this reason, our research is aimed at studying the presence of 12 different tolerance genes in the medicinal plant M. charantia acclimatized in Uzbekistan. Selected genes namely CSP3, CYP707A, GST, PYL4, BCH, POD1, RBOH, CAT, GR, PP2C78, PP2C8 and SOS2 stress tolerance including cold tolerance, drought tolerance, salinity tolerance, pathogens and cadmium known to impart various properties related to resistance to heavy metals such as. Understanding the presence of these stress tolerance genes is essential to elucidate the genetic adaptation of M. charantia to withstand multiple abiotic stresses.
Keywords: Momordica charantia, Abscisic acid, Setiltrimetil ammonium bromid, NanoDrop spektrofotometri
Cite this paper: Turgunov Jalaluddin Rahmonali Son , Naraliyeva Nasibakhan Mamanovna , Determination of Bitter Melon (Momordica Charantia L.) Genes Resistant to Different Stress Factors, International Journal of Virology and Molecular Biology, Vol. 13 No. 2, 2024, pp. 32-35. doi: 10.5923/j.ijvmb.20241302.03.
1. Introduction
- Momordica charantia L belongs to the Cucurbitaceae family and is a popular and widespread vegetable in Southeast Asia due to its edible and medicinal properties. This plant contains a lot of minerals, vitamins C and E, etc. [1]. It is also used to modulate blood glucose and treat diabetes in our daily life [2]. M. charantia contains important phenolic compounds that contribute to its antioxidant, antibacterial, and anti-inflammatory properties [3], and is used against gastric diseases, hypertension, obesity, and cancer [4].Due to the adverse effects of the environment, the growth and productivity of plants decreases. The sensitivity of plants to drought is a complex process that depends on various physiological and biochemical aspects. Prolonged drought stress damages photosynthetic processes, particularly chlorophyll pigments [5]. To combat this adverse abiotic stress, plants employ strategies such as producing abscisic acid (ABA) and accumulating suitable molecules such as s ugars and proline. They also develop enzymatic and non-enzymatic antioxidant systems to protect cells from oxidative damage [6].Low temperature is the main abiotic factor limiting plant germination, growth and spread [7,8]. When exposed to low temperature stress, plants usually develop various physiological and biochemical changes and modulate gene expression to achieve cold adaptation, for example, antioxidant enzymes produce antioxidants and osmotic solutions [9,10].Cadmium (Cd) is widely recognized as one of the most harmful heavy metals in soil. Acute toxicity, water solubility, non-degradability and extremely dangerous pollutant for living organisms. Studies have shown that Cd is more readily absorbed by many plant species than other heavy metals. Excessive accumulation of Cd2 + in plant tissues can cause serious phytotoxicity and have various negative effects on plant growth and development. These effects include destroying the chlorophyll structure of leaves, stopping photosynthesis and respiration, disrupting the absorption and translocation of mineral nutrients, accumulation of reactive oxygen species (ROS), suppressing protein synthesis and enzymatic activity, growth attenuation stops root growth [11].The main antioxidant enzymes that play a crucial role in scavenging reactive oxygen species (ROS): CAT (Catalase), SOD (Superoxide dismutase), PPO (Polyphenol oxidase), GPOD (Guaiacol peroxidase) and APOX (Ascorbate peroxidase) [12].
2. Material and Methods
- Plant Materials: Momordica charantia L is a plant belonging to the Cucurbitaceae family. Freshly grown plant samples were used. Samples were taken from undamaged leaves. Molecular research methods were used in the research. CTAB (cetyltrimethylammonium bromide) method is carried out in the following order.Stage 1. We measure 30 mg of plant leaf tissue on a scale, grind it in a mortar and add 200 µl (600 µl - 1000 µl) of STAB solution kept in a water bath and mix well.Stage 2. We put 0.5 ml of the homogenate into epindorffs, and then put it in a thermostat at 65°C for 90 minutes with occasional shaking (inverted).Stage 3. 600 µl of 24:1 mixture of chloroform(CHCl3)/ isoamyl(C5H12O) alcohol is added. Mix the homogenate for 5 sec. we catch in the vortex. 10 min. We put it in a centrifuge at a speed of 12000. In this, 3 different layers are formed, and DNA occupies the topmost layer.Step 4. Using a pipette, transfer 400 µl of the supernatant to a 1.5 ml Epindorf.Step 5. Repeat steps 3-4.Step 6. Add 40 µl of cold 3M sodium acetate (pH 5.2) and 400 µl of chilled isopropyl alcohol and shake several times. In this case, the DNA begins to sink down.Step 7. The supernatant is incubated in a refrigerator at -20°C for 3 hours (or left overnight).Step 8. The supernatant is removed from the refrigerator and centrifuged at 14,000 rpm for 10 min. DNA precipitates white. The part of the supernatant up to the DNA is removed.Step 9. To wash the DNA, add cold 70% ethanol in a volume of 900 µl and put it in a centrifuge for 15 min at a speed of 14,000. In this case, the DNA remains as a precipitate.Step 10. Ethanol is removed. 37°C for 20 min. is left open. DNA does not need to be overstretched.Step 11. Separated DNA is dissolved in 100 µl of TE buffer (pH=8), slightly heated and the concentration in the nanodrop is checked.
3. Result
- In recent years, the effects of drought on the characteristics of Momordica charantia L cultivars [13], researches were conducted on gene expression and metabolic changes of Momordica charantia L. seedlings in response to low temperature stress [14]. However, despite the fact that abiotic stresses such as drought, sudden temperature changes, salinity, and heavy metals can reduce plant growth and productivity by more than 50% [15,16], The presence of tolerance genes in the genome of M. charantia has not been sufficiently studied.For this reason, our research is aimed at studying the presence of 12 different tolerance genes in the medicinal plant M. charantia acclimatized in Uzbekistan. Selected genes namely CSP3, CYP707A, GST, PYL4, BCH, POD1, RBOH, CAT, GR, PP2C78, PP2C8 and SOS2 stress tolerance including cold tolerance, drought tolerance, salinity tolerance, pathogens and cadmium known to impart various properties related to resistance to heavy metals such as . Understanding the presence of these stress tolerance genes is essential to elucidate the genetic adaptation of M. charantia to withstand multiple abiotic stresses. Through DNA extraction and PCR amplification methods using specific genetic markers, we aimed to determine the presence of these tolerance genes in plant samples from Uzbekistan. The identification of these stress-related genes demonstrates the genetic adaptation of M. charantia to withstand salinity stress and multiple abiotic stresses common in its native environment. In addition, it provides valuable insights to support the acclimatization and cultivation practices of this economically important medicinal species and to exploit the medicinal properties of the plant.
4. Discussion
- Further characterization of genes expressing these stress tolerances will contribute to a comprehensive understanding of the genetic basis of stress tolerance in M. charantia. Our research may help develop strategies for sustainable cultivation and use of this medicinal plant, increasing its economic value and improving its medicinal properties. DNA was extracted from fresh leaves of M. charantia acclimatized in Andijan, Uzbekistan. Ensuring the reliability of experimental data, only undamaged and disease-free leaves were selected for further analysis. DNA was isolated from 20 mg of young leaf tissue using a modified CTAB (cetyltrimethylammonium bromide) method. This extraction process involves homogenizing the samples into a fine powder. The resulting powder was subjected to a series of steps including CTAB extraction, incubation, chloroform/isoamyl alcohol extraction, DNA precipitation and purification. The quality and quantity of the obtained DNA was evaluated using a NanoDrop spectrophotometer, and the suitability of the samples for further analysis was ensured (Fig. 1).
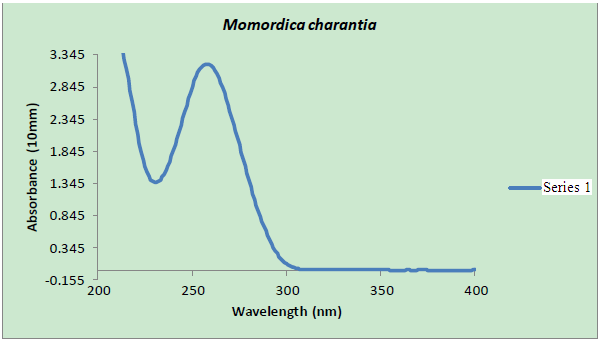 | Figure 1. The result of testing the quality and quantity of DNA extracted using the NanoDrop spectrophotometer. Concentration) 157.80 ng/ul, A260/A280) 2.289, A260/A230) 2.345 |
|
5. Conclusions
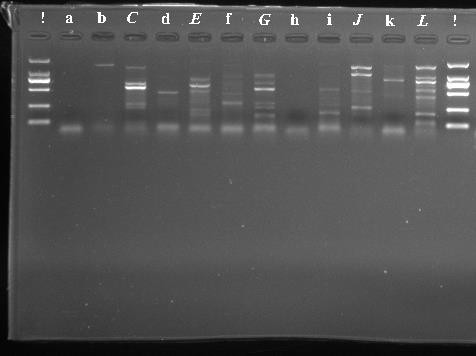
- Overall, the presence of these specific markers in gel electrophoresis results provides strong evidence for genetic adaptations and stress tolerance mechanisms present in M. charantia. These findings shed light on the plant's ability to thrive under harsh environmental conditions and may have important implications for increasing crop resilience and improving the sustainable cultivation of medicinal plants in adverse environments. Further study of these genetic traits and their underlying mechanisms will ensure the creation of stress -resistant crop varieties and the preservation of valuable plant species. This study shows for the first time that 253 CSP3, 263 GR, 273 POD1, 278 PYL4 and 279 RBOH specific genetic markers associated with abiotic stress tolerance are present in M. charantia using gel electrophoresis. Identification of these characteristics will provide an opportunity to develop stress-resistant varieties of M. charantia through marker-assisted breeding for sustainable cultivation under adverse conditions. Future work will include marker validation and assessment of stress response.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML