-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Virology and Molecular Biology
p-ISSN: 2163-2219 e-ISSN: 2163-2227
2024; 13(1): 8-14
doi:10.5923/j.ijvmb.20241301.02
Received: Mar. 14, 2024; Accepted: Apr. 12, 2024; Published: Apr. 17, 2024

Comparative Study of Two Sets of Primers MY09/ MY11 and GP5+/GP6+ Used for the Detection of Human Papillomaviruses Cervical Infections from Women Living in Yaounde-Cameroon
Grace W. Yimga1, 2, 3, Cyrille L. Kountchou2, 3, Urielle D. Seuga1, Vigan F. V. Codjo1, Mama III de Njifoutahouo W.1, Ebogo Belobo J. T.2, Edoun Ebouel F. L.2, Cyrille Mogo2, Bopda Waffo A.4, Tedom Jean-Claude1
1Department of Microbiology, Catholic University of Central Africa, Yaoundé, Cameroon
2Institute of Medical Research and Medicinal Plants Studies (IMPM), Yaoundé, Cameroon
3Department of Biochemistry, University of Dschang, Cameroon
4Department of Biological Sciences/College SMT1627 Hall Street Montgomery, AL, USA
Correspondence to: Grace W. Yimga, Department of Microbiology, Catholic University of Central Africa, Yaoundé, Cameroon.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

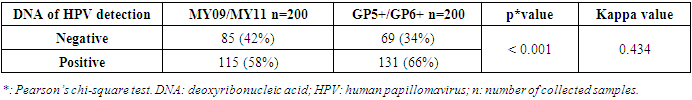
Introduction: Infection with Human papillomaviruses (HPV) has been demonstrated as a major cause of cervical cancer. Various methods are used to screen HPV infections. Concerning HPV DNA testing, there are issues on assessing the choice of primers for HPV detection. Even though HPV infections are identified in Cameroon, the choice of primers stays a puzzle. Various primer sets are generally used. The objective of this study was to compare the level of detection of HPV using two PCR primer sets, MY09/MY11 and GP5+/GP6+, and evaluate potential risk factors of HPV using cervical-vaginal fluid of women in Yaoundé. Material and Methods: Participants in this study came from “Centre Medical Catholique MarieReined’Etoudi” (CMCMRE) of Yaoundé and Centre d’Animation Social et Sanitaire (CASS)-Nkolndongo health centers. 200 women agreed to participate in this study. After having collected some social and cultural data with the aid of a questionnaire, cervical vaginal fluids were collected using the standard collection procedures and stored in a transport medium at -20°C. Then, DNA was extracted from these samples using the Cetyl Trimethyl Ammonium Bromide (CTAB) method. For each sample, two sets of primers were used: MY09/MY11 and GP5+/GP6+ by the conventional PCR method. Results: Regarding the amplification success rate, MY09/MY11 primer set gave 58% (115 of 200 participants) for the revelation of HPV DNA product (450 bp) while the GP5+/GP6+ primer set gave 65.5% (135 of 200 participants) for the revelation of HPV DNA product (150 bp). The concordance between the two primer sets gave 0.434 as kappa value. The lifetime number of sex partners was the only risk factor whose association was statistically significant with the primer set GP5+/GP6+ (p=0.004). Conclusion: This study suggests that, the GP5+/GP6+ primer set could be used as a primer of choice for the detection of HPV infection for better patient follow-up.
Keywords: HPV infection, MY09/MY11, GP5+/GP6+
Cite this paper: Grace W. Yimga, Cyrille L. Kountchou, Urielle D. Seuga, Vigan F. V. Codjo, Mama III de Njifoutahouo W., Ebogo Belobo J. T., Edoun Ebouel F. L., Cyrille Mogo, Bopda Waffo A., Tedom Jean-Claude, Comparative Study of Two Sets of Primers MY09/ MY11 and GP5+/GP6+ Used for the Detection of Human Papillomaviruses Cervical Infections from Women Living in Yaounde-Cameroon, International Journal of Virology and Molecular Biology, Vol. 13 No. 1, 2024, pp. 8-14. doi: 10.5923/j.ijvmb.20241301.02.
Article Outline
1. Introduction
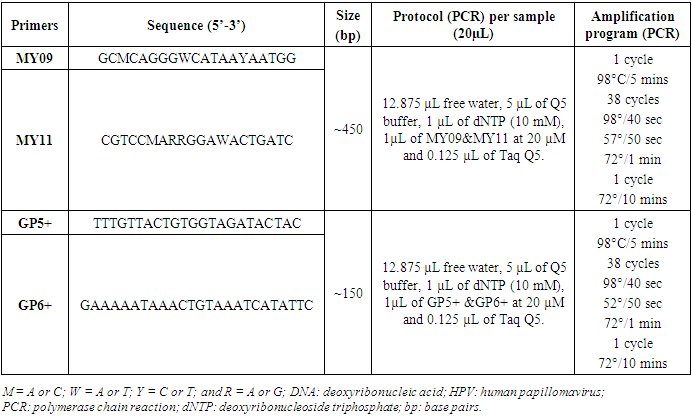
- Several diseases undermine our society today among these, cancer. Globally, cervical cancer is the fourth most common cancer in women, with 604 000 new cases in 2020 [1]. About 90% of the 342 000 deaths caused by cervical cancer occurred in low- and middle-income countries. The highest rates of cervical cancer incidence and mortality are in sub-Saharan Africa (SSA), Central America and South-East Asia [1]. The major cause of cervical cancer now recognized is persistent infection of Human papillomaviruses (HPV) [2]. HPV are viruses of the Papillomaviridae family. They are small, non-enveloped viruses that are 55 nm in diameter and are very resistant. The HPV genome constitutes a circular double-stranded DNA molecule [3]. More than 200 HPV genotypes have been identified, 112 (HPV1 to HPV112) of which were characterized after cloning and sequencing of their genomes [3]. The high burden of cervical cancer in developing countries are related to inequalities in access to vaccination, screening and treatment services [1]. In Benin, a total of 18 different HPV types were identified, with an overall prevalence of 33.2% [4]. In Cameroon a study had as findings, a frequency of 1000 new cases of cervical cancer per year among which 300 deaths and a frequency of the detection of 14 high risk HPV types at 30.1% [5]. Also, a global frequency of 24.3% of total HPV types was observed [6]. Several methods are used to determine cervical cell classification and/ or highlight precancerous lesions and not to detect the virus. These methods are: Papanicolaou test, liquid based monolayer cytology, visual inspection of the cervix using acetic acid (VIA) and visual inspection of the cervix using lugol’s iodine (VILI). HPV DNA testing method uses different techniques (Hybrid capture HPV DNA test 2/ hc2 and Polymerase Chain Reaction/ PCR) with the aim of detecting the genome of the virus if present. Human Papillomaviruses are non-cultivable and therefore there is no gold standard test to detect this virus. However, only HPV DNA testing method can be used. PCR as the most sensitive and specific technique, was that chosen for this study. The advent of PCR based methods for the detection of HPV infections has seen a huge improvement in HPV infection detection. Many reagents come into play among which primers. Different primers’ couples are used for the detection of the virus. Concerning HPV DNA testing, there are issues on assessing the choice of primers for HPV detection. The PCR system commonly uses MY09/MY11 and GP5+/GP6+ primers that amplify the L1 region of viral genome. They have been used to amplify the conserved regions of HPV genomes (L1 region). The widely used MY09/MY11 consensus primers set is synthesized with several degenerated (a nucleotide is incorporated at random at certain positions during synthesis) nucleotides in each primer and is thus a mixture of 25 primers, targets a 450 bp conserved sequence in the HPV L1 gene, and is therefore able to amplify a broad spectrum of HPV types. The GP5+ and GP6+ primer set consists of a fixed nucleotide sequence for each primer and detects a wide range of HPV types by using a lowered annealing temperature during PCR, and targets a 140bp sequence of HPV L1 gene, located inside the sequence recognized by the MY primers [7]. The GP5+/GP6+ primer set is an improved model of GP5/GP6 by addition of nucleotides at the 3’ ends of these primers. Figure 1 below illustrates the primer sets and the viral region they amplify.
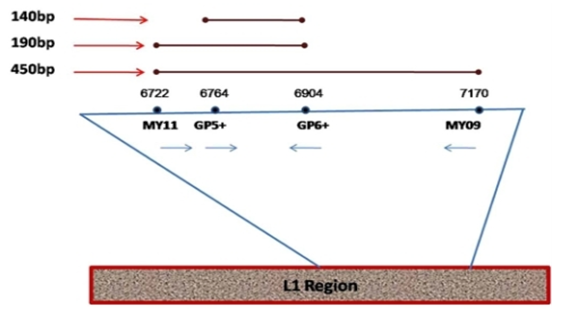 | Figure 1. Diagrammatic representation of the locations of MY09/11, GP5+/6+ general primer sets on L1 region of HPV genome. The expected amplicon size for each of the primer set is indicated [8] |
2. Material and Method
- Study area, data collection, ethical consideration and sample collectionThis is a cross-sectional descriptive and comparative study conducted in Yaoundé-Cameroon from July to December 2017 in the following hospitals: Centre Medical Catholique Marie Reine d’Etoudi (CMCMRE) and Centre d’Animation Social et Sanitaire (CASS) of Nkolndongo. The study included women coming to one of these hospitals, aged 15 and above who were sexually active, apparently healthy and who signed the informed consent or sexually active adolescents who provided their assent. Participants’ data were obtained from questionnaires which they filled. The questionnaires contained six items. Data collected comprised of: baseline data, gyneco and obstetrics data, HIV status and genital signs. Direct contact was made with each participant when the latter was filling the questionnaire. The aim of this direct contact was to ensure each participant understood each question in the questionnaire and to create an atmosphere of confidence. This study was approved by the Institutional Ethics Committee with registered number 2017/0573/CEIRSH/ESS/MIM as well as authorizations from the different health centres were obtained. Cervical swabs were collected and placed in Eppendorf tubes containing physiological water (pH 7.0) and stored in a transport medium at -20°C until DNA extraction.DNA extractionGenomic DNA was isolated from the samples using the CetylTrimethyl Ammonium Bromide (CTAB) method described by Murray and Thompson [9] with some modifications. 400 μL of samples were washed each with 500 µL of sterile distilled water and later digested with 500μL of CTAB buffer solution, transferred to a 60°C water-bath for 30 minutes. Following the incubation period, DNA was separated from the lysate by carefully adding 500 µL of chloroform: isoamyl alcohol (24v: lv). Only the aqueous phase was pipetted in another new pre-labelled Eppendorf tube. 500 µL of isopropanol was added in each tube Then, gently mixed manually. This mixture was conserved in a deep freezer at -20°C for 24 hours. Following this incubation period, the samples were removed from the deep freezer to let it cool. Then, centrifuged at 13 000 turns/minute for 15 minutes. The supernatant was discarded and the bottom conserved. 500 µL of absolute ethanol and 20 µL of sodium acetate solution were added for a second precipitation and conserved for 03 h at -20°C. Remove from the freezer, centrifuge at 13 000 turns/minute for 15 min. Discard the supernatant and conserve the bottom. Add 500 µL of 70% ethanol to remove excess salt by centrifugation. Centrifuge at 13 000 turns/ minute for 15 minutes. Discard the supernatant and conserve the DNA pellet (often invisible). Air dry DNA pellets at room temperature for 24 hours. Re-suspend DNA pellets in 30 µL of free water or PCR water then diluted 10-fold and stored at -20°C until amplified by PCR. To determine extracted DNA quality, each DNA was analysed by electrophoresis on a 1% agarose gel stained with ethidium bromide (Et-Br) (Figure 2).
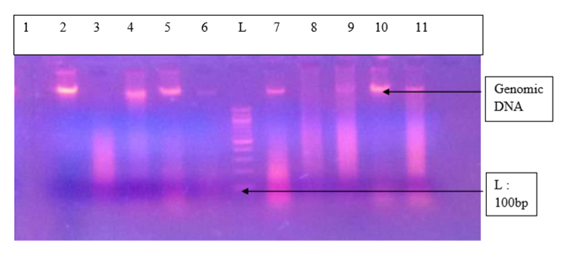 | Figure 2. Agarose gel (1%) stained with ethidium bromide showing genomic DNA extract. (Legend: L: 100 bp DNA ladder; DNA: deoxy ribonucleotide acid; 1 to 11 = DNA extract/ samples extracted) |
|
3. Results
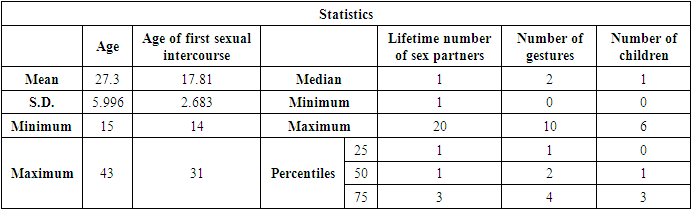
- Samples were collected from women attending the two different health centers (CMCMRE and CASS) between the periods of 28th August to the 20th of October 2017. In this study were enrolled 200 women whose quantitative baseline characteristics are described below (Table 2).
|
|
|
4. Discussion
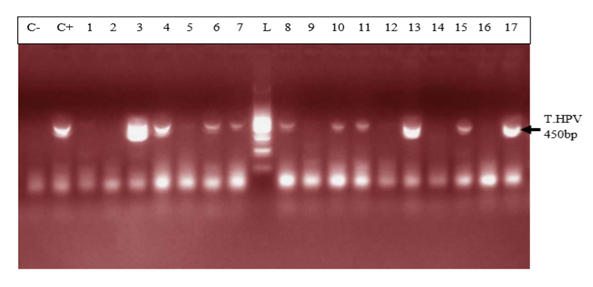
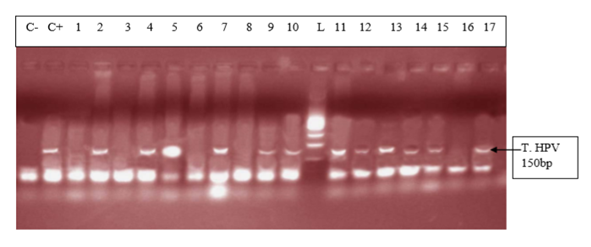
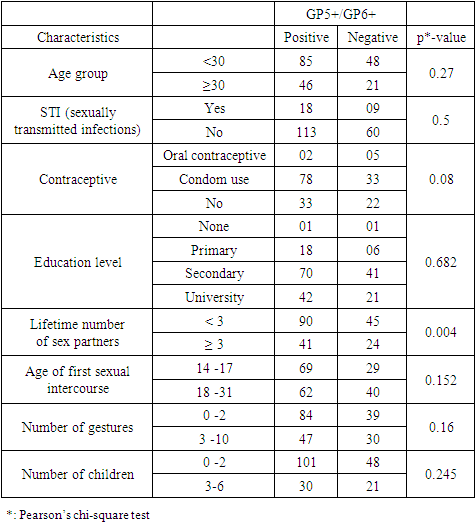
- The mean age in the study population was 27.3 ± 5.996 years with a variation from 15 years to 43 years. This is justified by the fact that it’s in this age group that we find women in intense sexual activity. The average age in this study (27.4 ± 6.4years) is less than that of the study published in Cameroon [5] which was 47 ± 11.8 years. The mean age of first sexual intercourse was 17.81 ± 2.68 years. This mean age is higher than that of a study in Cameroon [10] who had 15.5 years for females. The increase observed can be due to the fact that young girls nowadays are more educated and the rate of early marriages is reduced than in the past. The median lifetime number of sexual partners was 01 (min-max: 01-20); that of number of children was 01 (min-max: 0-6) and that of number of gestures 02 (min-max: 0-10). It was also observed that 14% of the study population reported previous infections with chlamydia as the most prevalent (63%). As concerns level of education, the secondary level was highly represented with 112 participants. This could probably be due to socio economic conditions such as poverty obliging young girls to drop from schools. 115 vaginal samples were tested positive for total HPV types with MY09/MY11, a prevalence of 58%. This result is much higher than that carried out in the United States [11] which was 42.5% of total HPV. This difference would probably be due to socio-economic conditions. When compared to the results of the studies in Ibadan-Nigeria [12] who found 40% and in Tunisia [13] who found 43.2% (mean age of 45.75); the high rate of our results would be due to the average age of the study population of 27 years. It was also higher than that found at Centre Pasteur du Cameroun [5], which showed a prevalence of 30% for total HPV types and also that declared by the National Committee for the Fight against Cancer (Comité National de Lutte Contre le Cancer, CNLCA) in Cameroon which was 23.5% in 2016 in a study including 1470 women. This difference would be due to the average age of the patients which was 47±11.8 years and 42.5 years respectively against 27.3 ± 5.996 in our study. In our study, the percentage of the population under 30 is 67%. In addition, 39% of HPV positive were detected in the age group < 30years while in the age group ≥ 30 years, 19% were positive for HPV infection.Also, total HPV types were detected with GP5+/GP6+ primer set on the same 200 cervical vaginal fluid samples and gave a prevalence of 66% (131/200). This result is greatly higher compared to that of the study [14] whose HPV positivity was 22.3% by GP5+/6+. This could be explained by the fact that the latter study included samples derived from five IARC-coordinated studies in Bhutan, Rwanda and Mongolia populations giving an uneven rate of HPV positivity across these countries. In Conakry, the study [15] gave an overall HPV prevalence of 50.8%. This result is less than that observed in our study and could probably be due to the fact that the greater part of women positive in Conakry-Guinea had cervical abnormalities (78.5%). In addition, 43% of HPV positive were detected in the age group < 30 years while in the age group ≥ 30 years, 23% were positive for HPV infection.These results are similar to the findings of Gargano [16] which detected HPV infection at 81.6% for participants aged< 30 and 57.6%,for those aged ≥30.115 (58%) were tested positive with MY09/MY11 while 131 (66%) were tested positive with GP5+/GP6+ showing that GP5+/GP6+ gives a higher positivity rate. Similarly, a study [17] reported HPV DNA detection in 15% and 20.7% of normal cervical samples, respectively, using MY09/MY11 and GP5+/GP6+ PCR, and in 37.1% and 50% of mild dysplasia samples, suggesting that GP5+/6+ primers may result in increased amplification efficiency for not containing any degenerate bases, and for cover a small region compared with MY09/11 primers. However, in a similar study, the authors do not find statistical difference (p-value = 0.3261). The HPV-DNA detection with MY09/11 primers was 15%, and 30% for GP5+/6+ primers used in PCRs [18]. From the p-value= 0.0000 of our study, there is an optimal difference in DNA HPV detection when using MY09/MY11 and GP5+/GP6+primer sets. The kappa value of these two primer sets is 0.434 which gives a moderate concordance between the two approaches. However, this kappa coefficient is not correct because in Biology it has to be greater than 0.8 in order to be correct. The risk of overestimating or underestimating HPV positivity, due to technical limitations and lack of gold standard are important aspects to consider [19]. Reason why it is recommended to use several methods or techniques for the detection of HPV.In most developing countries such as Cameroon, access to health services is limited and screening for HPV infection reaches few of the women who need it. A well-functioning health system, with the necessary equipment and trained providers, is essential for prevention activities, screening and diagnosis, linkages for follow-up and treatment, and palliative care [20]. We noticed that most of the infected HPV participants enrolled in our study were of the age group < 30 years. However, the most representative age group was 15-30 year old with an average of 27.3± 5.996 years old. This could be due to the fact that most people of such age are intensely sexually active. These results are similar to the study [16] which detected HPV infection at 81.6% for participants aged < 30 and 57.6%, for those aged ≥30. Based on GP5+/GP6+ results, the current study demonstrates an association with the lifetime number of sex partners (p-value = 0.004). This finding is in agreement with that of another study [21]. Also, the use of contraceptives could possibly be a significant risk factor (p-value = 0.08). However, condom use does not always prevent HPV transmission and data linking condom use with rates of transmission to a partner, or time to HPV clearance, are inconsistent [22]. There was no statistical significant association of the other risk factors (age of first sexual intercourse, number of gestures, and number of children, sexually transmitted infections (STI) and level of education with HPV positivity. This is similar to the findings of the study [23] at Bobo-Dioulasso in Burkina Faso. PCR is being increasingly used in clinical laboratories to HPV diagnose. Different primers for PCR have been developed, either type-specific or universal. Because of their lower cost and good analytical sensitivity, consensus primers are most frequently used in HPV detection for screening. Standardization and use of the GP5+/GP6+ PCR system could aid physicians in providing efficient screening and better treatment for patients. Our results show PCR using GP5+/GP6+ to be a more accurate means of establishing HPV prevalence rates and by so doing, there is a difference in their optimal detection which is statistically significant. Moreover, these results emphasize the importance of molecular diagnostic methods as a complementary tool to conventional preventive screenings.
5. Conclusions
- The positivity rate of HPV detection with MY09/MY11 primer was 58%; the positivity rate of HPV with GP5+/GP6+ primer was 66%; the overall concordance between the two approaches is 43.4%; there is a significantly higher performance in HPV amplification using GP5+/GP6+; based on GP5+/GP6+ results, number of sex partners and possibly the use of contraceptive, are leading risk factors of HPV acquisition in our context. From our point of view, this research gives the primer set that could be used in the screening of women for HPV infections during epidemiological researches and in the clinical domain since it has been recommended by FDA in the United States to associate PCR method and cytology for a better management of women.
ACKNOWLEDGEMENTS
- Grateful to all participants who helped to realize the study. Thanks goes to the research team members.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML