-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Virology and Molecular Biology
p-ISSN: 2163-2219 e-ISSN: 2163-2227
2024; 13(1): 1-7
doi:10.5923/j.ijvmb.20241301.01
Received: Mar. 12, 2024; Accepted: Mar. 28, 2024; Published: Mar. 30, 2024

Micropropagation of Wild Relatives of Cultivated Tulipa Species from Samarkand Region (Uzbekistan)
Shukrullozoda Roza Shukrullo Qizi1, 2, Kadirov Bakhtiyor Esanovich1, 2, Khaydarov Khislat Kudratovich2, Dekhkonov Davron Burxonovich3, Tojibaev Komiljon Sharobitdinovich4, Umurzakova Zebuniso Iskandarovna2, Shodiyeva Ozoda Majidovna5
1Uzbekistan in vitro Laboratory of Sag Agro "Bog'bon", Samarkand, Uzbekistan
2Samarkand State University named after Sharof Rashidov, Samarkand, Uzbekistan
3Namangan State University, Namangan, Uzbekistan
4Institute of Botany Academy Sciences of the Republic of Uzbekistan, Tashkent, Uzbekistan
5Navoiy State Pedagogical Institute, Navoi, Uzbekistan
Correspondence to: Shukrullozoda Roza Shukrullo Qizi, Uzbekistan in vitro Laboratory of Sag Agro "Bog'bon", Samarkand, Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Tulipa is bulbous geophytes with highly ornamental valueworldwide. Anthropogenic impact to the species led to decrease of wild Tulipa population including Uzbekistan. The impact challenges development of perspective conservation approaches. Thus, current work presents micropropagation of rare and endangered species T. fosteriana and Т. ingens distributed in Samarkand region. The study presents optimization of sterilization plant materials, culture media, and adaptation of newly regenerated plants by the use of bulb and seeds of selected taxa.
Keywords: Tulipa, in vitro, Microclonal propagation, Culture medium, Bulb propagation, Seed propagation, Germ culture, Growth hormones, Adaptation of regenerants, Soil substrate
Cite this paper: Shukrullozoda Roza Shukrullo Qizi, Kadirov Bakhtiyor Esanovich, Khaydarov Khislat Kudratovich, Dekhkonov Davron Burxonovich, Tojibaev Komiljon Sharobitdinovich, Umurzakova Zebuniso Iskandarovna, Shodiyeva Ozoda Majidovna, Micropropagation of Wild Relatives of Cultivated Tulipa Species from Samarkand Region (Uzbekistan), International Journal of Virology and Molecular Biology, Vol. 13 No. 1, 2024, pp. 1-7. doi: 10.5923/j.ijvmb.20241301.01.
Article Outline
1. Introduction
- Tulips (Tulipa L.) own considerable ornamental and aesthetic value worldwide. At the current time number of species of the genus is unknown due to difficulties in the taxonomy of the genus, confusion in the classifications, high rate of interspecific hybridization and polymorphism (Christenhusz et al., 2013). According to World Flora Online (2023), 376 scientific names related to Tulipa are presented and out of 85 (23%) accepted species, 266 (71%) synonymized taxa and 25 (7%) names have not been assessed yet. Currently, the primary center of diversity of wild Tulipa presents over 65 species (Dekhkonov et al., 2022a; Asatulloyev et al., 2023;), of which 33 species occur in Uzbekistan (Tojibaev et al., 2022). Despite the long history of Tulipa research in Uzbekistan on morphology (Botschantzeva, 1962; Vvedensky & Kovalevskaja, 1971; Dekhkonov et al., 2022a; Everett, 2013; Shukrullo qizi, 2023;), phytogeography (Tojibaev and Beshko, 2014; Dekhkonov et al., 2021;), states of the species under climate change (Dekhkonov et al., 2022b; Asatulloev et al., 2022), molecular studies (Christenhusz et al., 2013; Wilson, 2023), micropropagation of the species has not been studied (Shukrullozoda, 2022a; Shukrullozoda et al, 2022b) enough yet. The IUCN Red List includes more than 42,100 species threatened with extinction (IUCN, 2023). The rapid growth of the population (Statistics Agency under the President of the Republic of Uzbekistan, 2023) and economy lead to an over-exploitation of natural capital, an increase of urbanization rate and fragmentation/loss of natural habitats. Indeed, 187 species (60%) out of the 314 red-listed taxa of the flora (7.13%) require special protection measures or are not protected at all. Currently, 19 species of Tulipa included in Red Data Book of Uzbekistan (Khasanov, 2019) including T. fosteriana and T. ingens. Main threats of these species are overgrazing, land use for agricultural purposes and overexploitation. One of the effective conservation approaches is micropropagation of plant species (Dekhkonov et al. 2023; Shukrullozoda, 2023;). in vitro tissue culture techniques are considered one of the effective approaches of conservation of rare and endangered species. There are sufficient contributions on scientific works of Wright N.A. и Alderson P.G. (1980); Gabryszewska, E. And Saniewski, M. (1982); Alderson et al. (1983); B. Aubert, G. Weber and N. Dorion (1985); Nishiuchi Y. (1986); Hulscher M et al. (1992); Kuijpers and Langens-Gerrits (1997); Y.D. Sharma and Shiv Bhushan Kanwar (2003); Minas (2007); Aхметова A.Ш. (2009); Musadiq Hussain Bhat et al. (2020); Podwyszyńska and Marasek-Ciolakowska (2020) in vitro micropropagation of Tulipa species. However, in vitro micropropagation of wild relatives of cultivated Tulipa species distributed in Uzbekistan has not been developed. Hence, the purpose of the work is to develop micropropagation of wild relatives of ornamental and red-listed species of T. ingens and T. fosteriana as the effective conservation effort.
2. Material and Methods
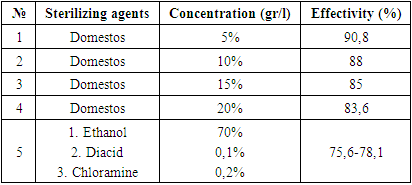
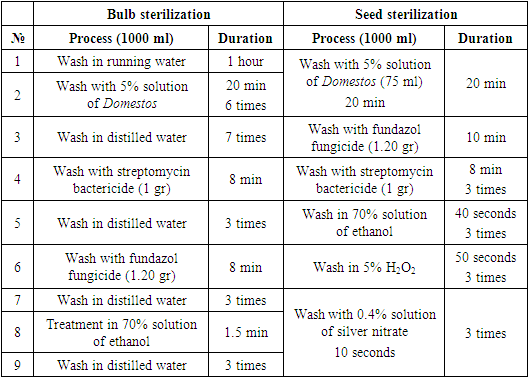
- Study area. Samarkand is one of the oldest regions of the Republic of Uzbekistan located at the southeast of the country with 123.8 km2 total area. The region bordered Kashkadaryo (in the south), Bukhara (in the west), Djizzakh (in the east) and Navoi (in the northwest) regions. In terms of biogeography Samarkand is located on the western part of the Pamir-Alay mountains, in the middle of the Zarafshan river. The area is surrounded by Nurata and Aktau mountains of the Turkestan mountain ranges from the north and Zarafshan mountain ranges from the south. The climate is continental. Average annual precipitation is about 282-460 mm. Plant material and sterilization. Bulbs and seeds of T. fosteriana and T. ingens were collected from the vicinities Omonquton and Nurabad district.Sterilization of the bulbs were sterilized by Wright et al. (1980) (3), and Zaitseva (2015) (Zaitseva 2015) which are modified through the study. One bulb of the selected taxa was divided into 2-4 part for the direct formation of callus. Purified and sterilized seed was incubated in optimized culture medium.Sterilization of plant materials. Optimization of the sterilization of plant materials conducted at “in vitro Sag Agro Bog’bon” laboratory (Samarkand, Uzbekistan). Selection and optimization of sterilization agents were presented in table 1.
|
|
|
|
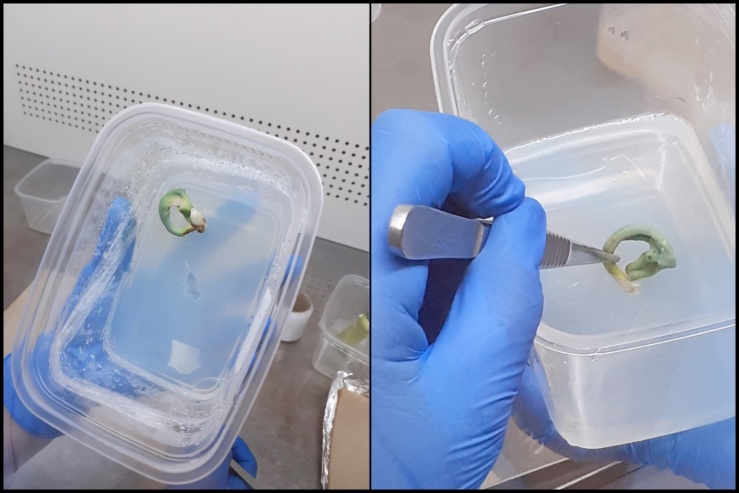 | Figure 1. Development of callus and organogenesis from bulb slices of Tulipa species by the use of Benzoaminopurine (0.5 mg/l) |
 | Figure 2. Adaptation of rooting of shoots to soil conditions |
3. Results
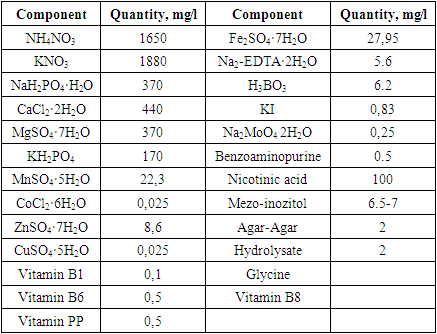
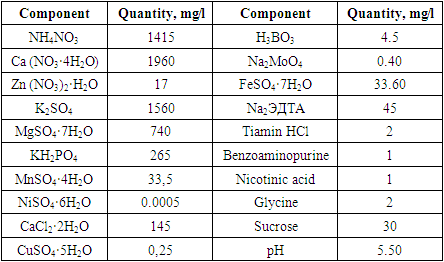
- According to the results, sterilization of the plant materials with 5% concentration of Domestos found to be the most effective and applied in further studies. Totally, the sterilization sequence contained 9 stages which included 5 times of treatment with fungicides and bactericides. Regime for isolated samples from was conducted depending on the type of explant. The presented sterilization approach of the seed and bulb slices of Tulipa showed as a perspective method for the scale up processes. Three criteria for the identification of the effectivity of the sterilization process were selected: number of infected (1) and necrotic (2) of planting samples and number of viable explants (3) after the sterilization process. Due to results, maximal number of viable explants (%), minimal infected (%) and necrotic (%) explants were obtained after the treatment of the bulb slices Domestos (5%) during 15 min and in the ethanol (70%) during 2 min. The effective optimization approach for the bulb sterilization allowed to obtain high percentage of viable explants.Viability of seeds and growth rate in the ground is about 23-39% (Botschantseva, 1962) and the rate was increased up to 60-96% after cold stratification process. In our study, stratification of seeds conducted at 6°С during 60-78 days. In this condition seeds of T. ingens and T. fosteriana begun to germinate after 35 and 41 days respectively. The growth effectivity of seeds was high for both species (T. ingens – 88% and T. fosteriana – 92%). After 6-7 months of cultivation 62.5% of explants were viable. Under in vitro conditions, each tulip shoot has an apical meristem surrounded by the base of a single curled leaf. After cooling process intensive growth of the leaves was observed. The first morphologic character in the bulb formation process is cessation of leaf growth, swelling of the bases of shoots and their progressive yellowing (Novak et al., 2014).Thus, in our study, seed growth was observed after 116 days, and the visible appearance of seed sprouts could be seen after 131 days (fig. 3.). Our results showed that eight shoots and accordingly, eight microbulbs were obtained from one embryo in the culture medium with 50-180 seeds in a capsule.In some approaches the leaves were sprayed with a 50% aqueous solution of glycerin or a mixture of paraffin (fat) and diethyl ether throughout the entire acclimatization period (1:1). The use of the approach provided 100% survival rate of regenerants. In our study, we did not use any of glycerin or a mixture of paraffin (fat) and diethyl since the humidity in the greenhouse is automated and varies between 40 and 60%. The system itself provides air humidity depending on seasonal changes. For example, in summer this system works intensively and sprays the air with water in the form of steam, and in winter the intensity is reduced due to the natural humidity of the environment. In addition, according to literature data, 20-30 days after planting, plants should be provided with mineral complexes at a temperature of 24°С. However, such work is not carried out, since the soil itself is rich in all mineral salts and fertilizers. In the future, as regenerants grow and depending on their salt needs, regenerants are impregnated with the necessary vitamins and mineral salts. But if the regenerate grows naturally and qualitatively, then no fertilizers are added to the soil and the whole process is trusted to natural growth.
 | Figure 3. The growth of seeds of T. fosteriana and T. ingens |
4. Discussion
- Podwyszyńska in their research work (2020) used a method of somatic embryogenesis. This method for tulip regeneration via somatic embryogenesis (SE) was developed using flower stem slices from cooled bulbs. These slices were cultured in darkness on modified MS media with auxins (2,4-D, NAA, and picloram) alone or with TDZ at 0.1 and 0.5 mg L−1. Callus formation occurred on the incised surface, leading to embryogenic callus lines. TDZ enhanced somatic embryo production, with the best results obtained using 2,4-D at 0.1 mg L−1 and TDZ at 0.5 mg L−1. Proline addition improved callus proliferation or embryo formation. High-quality embryos, capable of bulb formation, were observed with BAP at 0.1 mg L−1 instead of TDZ (7).The study investigates a sterilization method for Tulipa seeds and bulb slices using a 5% Domestos solution, which proved to be the most effective among tested protocols. This method, comprising nine stages including five treatments with fungicides and bactericides, is highlighted for its potential in scaling up processes. Sterilization effectiveness was gauged through three criteria: the number of infected and necrotic samples and the number of viable explants post-sterilization, where treatment with Domestos followed by ethanol significantly increased the viability of explants. Additionally, cold stratification of seeds at 6°C for 60-78 days markedly improved growth rates for T. ingens and T. fosteriana, showing an increase in seed viability up to 60-96%.Furthermore, the study explores in vitro cultivation outcomes, where a 62.5% viability rate was noted post-cultivation, alongside the development of morphological indicators of healthy growth. The research also reports on the germination and growth processes, where seeds showed visible sprouts after 131 days, leading to successful shoot and microbulb formation. This study suggests the efficiency of the sterilization process and cold stratification in enhancing the viability and growth of Tulipa, potentially applicable to other plant species. The innovative approach of relying on automated greenhouse humidity control further emphasizes the possibility of minimizing external intervention, leaning towards a more natural growth process.For a detailed analysis of the germination of the studied species in the external environment, further studies will be required to ensure the availability of wild species for germplasm collections.
5. Сonclusions
- Regarding seed viability and growth in soil, previous research (Botschantseva, 1962) reported viability rates of 23-39%, increasing to 60-96% after cold stratification. In our study, we conducted seed stratification at 6°C for 60-78 days, resulting in germination rates of 88% for T. ingens and 92% for T. fosteriana.We also optimized the culture medium, ensuring sterility through autoclaving and adding vitamins. The medium contained 4.5 µM Benzylaminopurine and 5 nM IBA for rooting before transferring to the greenhouse. After 6-7 months, 62.5% of explants remained viable under in vitro conditions. Micropropagation from seeds yielded successful growth, with eight shoots and microbulbs from one embryo.The transition of regenerated plants to non-sterile soil involved careful steps, including root washing and using a soil substrate of 70% peat and 30% vermiculite. Adequate illumination and humidity during acclimatization were critical for adaptation.Sterilization of the plant materials, optimization of culture medium, in vitro propagation protocols and adaptation techniques were developed for the selected taxa. We believe that our results will serve as a valuable data for ex situ conservation measure some red-listed Tulipa species in the future.
ACKNOWLEDGEMENTS
- I express my gratitude to the in vitro laboratory of Sas Agro "Bog'bon" for the support and provision of highly qualified assistance in conducting research on the microclonal reproduction of the studied objects.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML