-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Virology and Molecular Biology
p-ISSN: 2163-2219 e-ISSN: 2163-2227
2023; 12(2): 31-34
doi:10.5923/j.ijvmb.20231202.04
Received: Jul. 3, 2023; Accepted: Jul. 25, 2023; Published: Aug. 29, 2023

The Determination of the Titers of Polyclonal Antibodies Obtained to Potato X Virus Using Elisa
Dilfuza Jovlieva1, Abdirasul Vakhobov1, Vokhid Fayziev2
1National University of Uzbekistan, Tashkent, Uzbekistan
2Doctor of Biological Sciences, Associate Professor, Chirchik State Pedagogical University, Chirchik, Uzbekistan
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In this article, the process of titring polyclonal antibodies against potato virus X using elisa was studied. It is no secret that among the phytoviruses, the viruses infecting the potato plant are becoming more and more widespread and their epiphyticity is increasing. Timely detection of viruses requires the use of sensitive methods. IFA is such a method, it is based on the relationship between antibody (Ab) and antigen (Ag), where the titer of Ab is important. In this article, the titer of polyclonal Ab to potato X virus (PXV) isolated in Uzbekistan was determined using the DAS–ELISA method. For this, PXV antigen was isolated from the plant Datura tatula L. and virus–specific serum was prepared by means of rabbit immunization, and the prepared Ab titer was found to be 10–5 when determined by the sandwich variant of the enzyme immunoassay method. This allows the detection of the virus up to 10 μg.
Keywords: Potato X virus, ELISA, Antigen, Antibody, Conjugate, Phytovirus, Determination of the serum
Cite this paper: Dilfuza Jovlieva, Abdirasul Vakhobov, Vokhid Fayziev, The Determination of the Titers of Polyclonal Antibodies Obtained to Potato X Virus Using Elisa, International Journal of Virology and Molecular Biology, Vol. 12 No. 2, 2023, pp. 31-34. doi: 10.5923/j.ijvmb.20231202.04.
Article Outline
1. Introduction
- Human health can be preserved and included among the serious puzzles facing humanity since ancient times. If we take into account that the number of people in the world exceeds 8 billion, and the number of people suffering from hunger is close to 9 million [24], the way to solve the food problem is to increase nutritious crops, damage to agricultural plants it is necessary to prevent the causing factors, search for the cause and search for ways to fight against them. The increase in the mutual export of agricultural products leads to the spread of plants, an increase in the level of disease of plants, and the emergence of new strains and isolates of viruses due to external factors. The potato plant, which is considered as one of the agricultural food plants, belongs to the group of plants that are highly infected by phytoviruses, and it is infected with 52 of more than 400 known viruses, including 36 common viruses [1]. Among the studied phytoviruses, 7 viruses (PLRV, Y, X, A, S, M, AMY) were found to be dangerous for potatoes, and potato X virus (PXV) was included among the 4 extremely dangerous viruses among them [1]. In addition, PXV, together with other viruses, causes an increase in the level of disease of potatoes. Transmission of PXV to plants has been determined mechanically, through insects and soil, and even through containers containing seedlings in greenhouse conditions [3,4]. It has been found that this virus causes scattered mosaic and simple mosaic symptoms on plant leaves and coexists with other viruses in a latent (hidden) state [5,6].Several methods have been developed for the detection of viruses, and among the methods used for the diagnosis of viruses, immunological methods are distinguished by the fact that they require little work and time, and can examine several samples at the same time [7,8,9,10,11].The conducting such an analysis is based on the interaction of antigen (AG) and antibody (AT). One of these methods is immunoenzymatic analysis (IFA), which is also known as a process based on two components–an immune response and an enzymatic reaction.If the immune reaction attempts to identify biological molecules, cell or microorganism elements by forming a bond, the enzymatic reaction allows to see the result of the immunological reaction. That is, the immune response is actually a part of a complex process that determines the desired antigen [6,12,19].Immune reactions are specific reactions of binding between antigen and antibody with the formation of an immune complex. In enzyme immunoassay and any other immunochemical analysis, the primary process is the step of “recognition” of the analyzed compound by its specific antibody. Since the process of formation of immunochemical complexes occurs in a strictly quantitative ratio due to the concentration of components and reaction conditions, the initial concentration of the analyzed compound is determined by quantitative determination of the formed immune complexes [11,12].A number of Uzbek scientists, including V.B. Fayziev, A.H. Vakhobov, conducted a series of experiments to identify isolates of potato viruses found in Uzbekistan and study some of their characteristics using various methods. In particular, the effect of isolate PVXN–UZ 915 on the pigment content of D. stramonium plant was studied [16,17]. The necrotic isolate of PXV was found to be closely related to isolate GU384732.1 isolated in Australia based on PSR analysis and phylogenetic analysis [18]. Initially, it was determined that the specific serum for PXV and its titer was 1:16 by the double immunodiffusion method [19]. In addition, the spread of potato viruses X and L in reservoir plants and in the Samarkand region of Uzbekistan was studied using ELISA [20].D. stramonium, N. glutinosa, plants such as N. tabacum burley were used [1,6,21]. Breeding of PXV D. stramonium var. stramonium (green shoot and white flowers) this was done in the plant because it showed symptoms of systemic disease very quickly in a clear mosaic state [2,19].In some countries, hot sunny days limit the possibility of working with phytoviruses, because it is difficult to observe the symptoms of viruses in plants when the temperature is high. Therefore, when extracting PXV, D. stramonium var. tatula (red shoot and purple flowers) advantages of plant over other indicator plants were found, this plant was used to isolate the virus, and physiological changes in the plant under the influence of the virus were studied [22,23].Due to the high sensitivity of immunological diagnostics for the detection of viruses and for the purpose of using them in the identification of viruses, it was aimed to determine the specific antibody titer obtained for PXV by the IFA method.
2. Material and Methods
- Necessary reagents and equipment: to carry out the research, specific polyclonal serum obtained from PXV spread in the territory of Uzbekistan [22], antigen (D. stramonium var stramonium, D. stramonium var tatula) from the leaves of potatoes infected with the virus (Figure 1) amplified PXV (Figure 2)), conjugate (Antirabbit Ab+peroxidase), substrate (TMB), stop solution, Wash buffer (1% BSA) and polystyrene plates were used. In determining the results, IFA was determined from the analyzer and by visual inspection.
 | Figure 1. Potato leaves infected with PXV. a) sick b) healthy |
 | Figure 2. The leaves are mechanically infected with PXV. c) D. stramonium L., d) D. tatula L |
3. Performing a Diagnostic Test
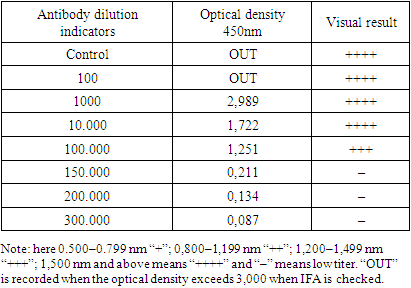
- First, Ab (Antibody) was diluted 1:100, 1:1000, 1:10,000, 1:100,000, 1:150,000, 1:200,000, 1:300,000 times. Specific Ab obtained for the virus was added to the polystyrene plates and left overnight at+4°C for 24 hours. After washing the excess Ab with 1% BSA, Ag was added and sorbed at 37°C for 1 h. After washing off excess Ag, Ab was placed on top of Ag and kept at 37°C for 1 h. After washing off excess Ab, it was added to Conjugate (Antirabbit Ab+peroxidase) and sorbed for 1 hour at 37°C. Excess Conjugate was also washed and TMB (tetramethylbenzidene) was added and kept in a thermostat for 1 hour at 37°C. After washing, the stop solution was added and after 20 minutes at 37°C in a thermostat, the result was visualized in an IFA reader at 405 nm.
4. Results and Discussion
- It is important to determine the polyclonal serum specific for PXV. The reason is that determining the sensitivity of the necessary reagents in the course of the experiment determines the quality of the diagnostic. In order to determine the developed method, the experiment was carried out in the case where polyclonal serum was diluted in different amounts. Other reagents were diluted accordingly. The time and temperature for sorption were chosen at different levels. As a result of the conducted experiments, the antibody titer was determined based on the optimal variant of DAS–ELISA. The flow chart of the DAS–ELISA method used to determine the specific polyclonal Ab titer obtained for KXV is given below (Figure 3).
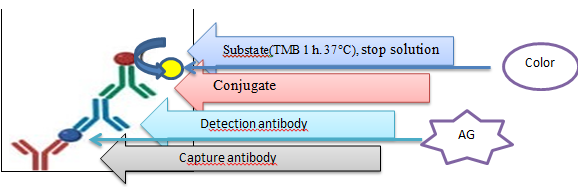 | Figure 3. DAS–ELISA test scheme |
|
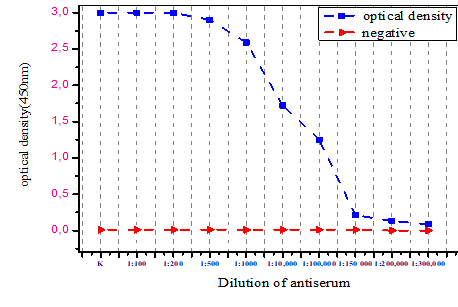 | Figure 4. Graphic representation of polyclonal anibody titer obtained for PXV detected in DAS–ELISA |
5. Conclusions
- As a result of the experiment, the polyclonal Ab titer obtained for KXV was found to be 100,000 (10–5). Therefore, the Ab obtained for KXV can be used for detection of viruses in a 10–5–fold dilution. Further continuation of the experiment allows to increase the antibody titer and use it in immunological methods. The authors express their gratitude to the staff of the Institute of Chemistry of Plant Substances for their assistance.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML