-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Virology and Molecular Biology
p-ISSN: 2163-2219 e-ISSN: 2163-2227
2023; 12(1): 7-12
doi:10.5923/j.ijvmb.20231201.02
Received: Mar. 15, 2023; Accepted: Mar. 29, 2023; Published: Apr. 15, 2023

Oil Degradation by the Strain Rhodococcus sp. HN4 with the Application of a Balanced Methanogenic Association of Microorganisms under Thermophilic Conditions
Barno Alimova1, Ozadokhon Pulatova1, Sherzod Tashbaev2, Mansur Sharifov1, Askar Kholikov1, Tokhir Mirzaev1, Tokhir Ishankhodjaev1, Akhmadjon Makhsumkhanov1, Kakhramon Davranov3
1Enzymology Laboratory, Institute of Microbiology of the Academy of Sciences of the Republic of Uzbekistan, Tashkent, Uzbekistan
2Andijan State University the Republic of Uzbekistan, Tashkent, Uzbekistan
3Director of the Institute of Microbiology of the Academy of Sciences of the Republic of Uzbekistan, Institute of Microbiology of the Academy of Sciences of the Republic of Uzbekistan, Tashkent, Uzbekistan
Correspondence to: Barno Alimova, Enzymology Laboratory, Institute of Microbiology of the Academy of Sciences of the Republic of Uzbekistan, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

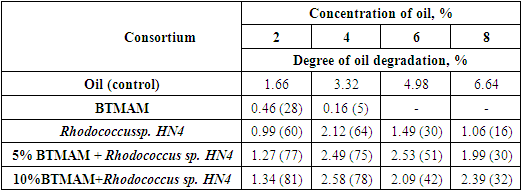
This article presents the results of a study on the possibility of intensifying the process of destruction of oil and oil products by a balanced methanogenic association of microorganisms (obtained after fermentation under thermophilic conditions) (BTMAM) by introducing into them a strain of the bacterium Rhodococcus sp. HN4. The amount of inoculum of Rhodococcus sp. HN4 was 4% of the volume of the inoculated medium. The BTMAM used in the experiment was 5 and 10%. Oil with a density of 832 kg/m3 (20°C) at a concentration of 2% to 8% was used as a substrate. Cultivation was carried out in flat-bottom flasks with a capacity of 250 ml on a shaker (150 rpm) at a temperature of 28–30°C for 20 days. Thus, it has been established that the bacterial strain of the genus Rhodococcus, possessing a wide range of metabolic capabilities, contributes to other groups of microorganisms that are part of BTMAM to assimilate hard-to-reach substrates, in particular oil and its decay products. BTMAM in the community with the Rhodococcus sp. HN4 degrades oil with a high degree of degradation and in high concentrations of 6-8%. The spectrum of microorganisms that make up the association are not pathogens; they are producers of biologically active compounds, resistant to high oil concentrations, in the oil degradation process, and contribute to soil biologization.
Keywords: Oil, a balanced association of microorganisms, Degradation, Microorganisms as oil degraders
Cite this paper: Barno Alimova, Ozadokhon Pulatova, Sherzod Tashbaev, Mansur Sharifov, Askar Kholikov, Tokhir Mirzaev, Tokhir Ishankhodjaev, Akhmadjon Makhsumkhanov, Kakhramon Davranov, Oil Degradation by the Strain Rhodococcus sp. HN4 with the Application of a Balanced Methanogenic Association of Microorganisms under Thermophilic Conditions, International Journal of Virology and Molecular Biology, Vol. 12 No. 1, 2023, pp. 7-12. doi: 10.5923/j.ijvmb.20231201.02.
Article Outline
1. Introduction
- Advancements in the oil industry have caused environmental pollution resulting from the components of oil and oil products that are toxic to living organisms. Solutions are needed to mitigate pollution and make the oil industry more environmentally sustainable. One solution to this problem involves the development of effective, environmentally friendly, and safe methods of purification using biological products based on microorganisms. Compared with some physicochemical methods, bioproducts based on microorganisms are ecologically safe and effective (Silva-Castro G. A. et.al., 2014, Dados A. et.al., 2015). The effectiveness of the use of biological products in solving environmental problems also depends largely on environmental conditions (temperature, pH, humidity), and the degree of the pollutant. During degradation, it is necessary to maintain the appropriate concentration of nutrients, especially nitrogen, and phosphorus, in an optimal ratio; this is necessary to compensate for the imbalance caused by the high carbon content of crude oil during pollution, which can slow down the growth and activity of microorganisms (Bamforth et.al., 2005, Ayotamuno et.al., 2006).Crop and livestock waste can be used to tackle the problem of environmental pollution from the oil industry. For example, several studies have shown the potential for using plant organic waste, such as sunflower and rice husks (Li P. 2002), banana peels (Agbor R. B. 2012), and husks from cocoa pods (Agbor R. B. 2012), as well as organic animal waste, including cow, goat, and poultry manure (Adesodun J.K et.al., 2008, Nwogu T. P. et.al., 2015, Agarry S.E. et.al., 2013). The microorganisms contained in organic waste possess broad enzymatic capabilities, and they have a bio-stimulating effect on the microflora of contaminated areas, thereby contributing to the solution of environmental problems (Delegan Y. A. et.al., 2016).The purpose of the work is to investigate the possibility of intensifying the process of destruction of oil and oil products by a balanced methanogenic association of microorganisms obtained after fermentation by introducing into them a strain of the bacterium Rhodococcus sp. HN4.
2. Materials and Methods
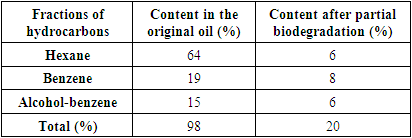
- To degrade the oil, the Rhodococcus sp. HN4 strain, which was isolated from soils contaminated with motor oil in a consortium with a balanced thermophilic methanogenic association of microorganisms (BTMAM), was obtained by successive fermentation of cattle manure and poultry manure (Tashbaev Sh.A. et.al., 2020). When studying the influence of the strain Rhodococcus sp. HN4 on the degree of oil degradation using a balanced methanogenic association of microorganisms obtained after fermentation, Rhodococ cultured on tryptone soy broth medium (HiMedia, India) was used for 48 hours. The amount of inoculation of Rhodococcus sp. HN4 introduced into the growing medium was 4% of the volume of the inoculated medium. BTMAM, which was used in the experiment, was previously diluted in sterile saline, and at a concentration of 5 and 10% was introduced into the medium. The moisture content of the applied BTMAM was 85–90% and pH = 7.6–7.9. Oil with a density of 832 kg/m3 (20°C) was used as a substrate. The oil was pre-sterilized in an autoclave for 30 min at 1 atm; after sterilization, at a concentration of 2% to 8%, it was added to Raymond's medium. BTMAM without the addition of Rhodococcus sp. HN4 was used as a control.The cultivation was carried out in flat-bottomed flasks with a capacity of 250 ml on a shaker (150 rpm) at a temperature of 28–30°C for 20 days. After 20 days of cultivation, a dilution was made and the titer of microorganism cells was determined using the Koch method (Netrusov A.I. et.al., 2005). Nutrient agar (HiMedia, India) was used to calculate the total number of cells of the microbial association. Crops were incubated at 28–30°C for 5–6 days.The degree of oil degradation was determined using the gravimetric method in relation to the total loss of oil in a liquid medium (Leonenko I.I. et.al., 2010). The analysis of the content of oil products in the medium was carried out as follows: After degradation, the samples were extracted with 50 ml of chloroform by shaking for 30 min. The chloroform extract was separated on a separating funnel and dried in a ventilated thermostat at a temperature of 70–75°C for 3–4 hours; it was then kept at room temperature for 18 hours, followed by weighing. The fractional composition of residual oil was assessed by chromatography using microcolumns filled with L40/100 silica gel (0.7 g each; Chemapol, Czech Republic), and the column was equilibrated with hexane. Samples of 0.005 g each, obtained by extraction during weight analysis, were dispersed in 1 ml of hexane, then applied to microcolumns and left for 20 min. Elution was carried out sequentially with hexane, benzene, and a benzene-ethanol mixture at a ratio of 1:1; 4 ml samples were then collected in tared test tubes. The resulting hexane, benzene, and benzene-alcohol fractions were evaporated in air and brought to constant weight in a ventilated drying cabinet at 75°C. The content of the components in the fractions was determined in reference to the weight of the initial sample. Finally, the genus and species affiliation of some strains belonging to the BTMAM association were determined from the spectrum of ribosomal peptides on matrix-assisted laser desorption/ionization–time of flight (MALDI-TOF; Bruker Daltonics, USA).
3. Results and Discussion
- The introduction of microbial associations to increase the efficiency of oil biodegradation is a promising and environmentally friendly method of remediating oil pollution in the environment. For active oil degradation, a combination of bacterial strains with wide enzymatic capabilities is required (Delegan Y. A 201653, Tashbaev Sh.A. et.al., 2020, Leonenko I.I. et.al., 2010, Ivanova A.A. et.al., 2015). It is known that bacteria of the genus Rhodococcus have a wide range of metabolic capabilities. They synthesize enzymes (dehydrogenases, peroxidases, oxygenases, alkyl sulfatase, nitrile hydratase, and phenol hydrolase) with broad substrate specificity and resistance to toxic compounds. Bacteria of the genus Rhodococcus are active biodegradants of hydrocarbons (aliphatic, aromatic, polycyclic, and heterocyclic) and their derivatives, which are difficult to access and toxic to other microorganisms. They are active producers of biosurfactants that emulsify oil and oil products and increase the bioavailability of pollutants. Currently, they form the basis of many biological products for cleaning oil pollution (Leonenko I.I. et.al., 2010, Netrusov A.I. et.al., 2005).Given the characteristics of the Rhodococcus genus described above, the ability to degrade oil in high concentrations (2%–8%) of the Rhodococcus sp. HN4 strain in conjunction with BTMAM was studied in this research. Previously, in the process of fermentation of chicken manure in thermophilic conditions, BTMAM was obtained, the microbial landscape was studied, and it was shown that the composition of BTMAM includes methane-forming, facultative, anaerobic, cellulose-decomposing, and nitrogen-fixing microorganisms. The number of which was: methane-forming 2.5*104, cellulose-decomposing 2.5*104, and denitrifying bacteria 5*103, with the content of ammonifiers 4*103. Since BTMAM was obtained in the process of thermophilic fermentation, it does not contain bacteria, pathogens belonging to the Enterobacteriaceae family, including the species Escherichia coli, the genera Shigella and Salmonella, as well as other pathogenic bacteria (Fig. 1).
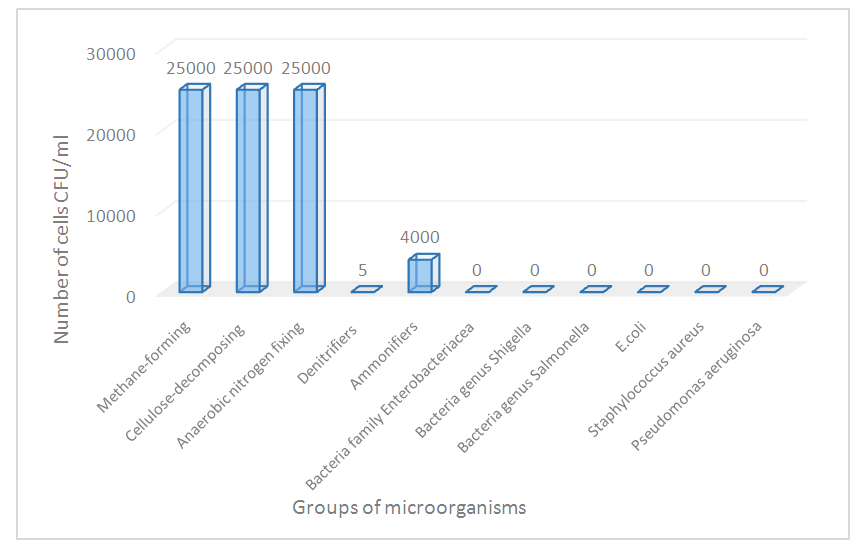 | Figure 1. The main groups of microorganisms constitute the BTMAM |
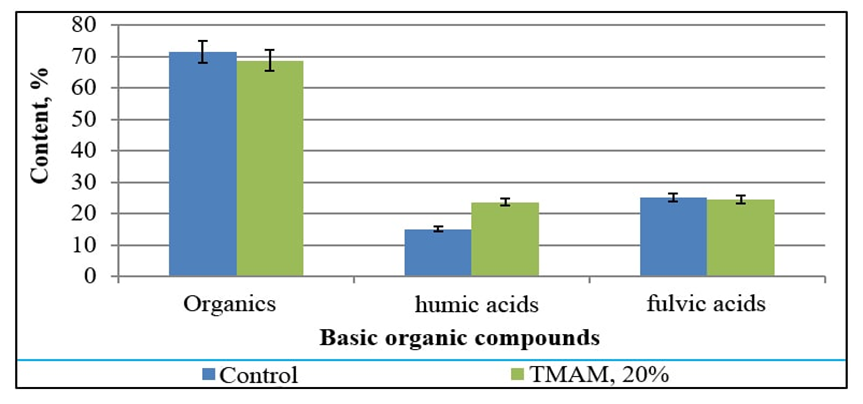 | Figure 2. The organic composition of the obtained BTMAM |
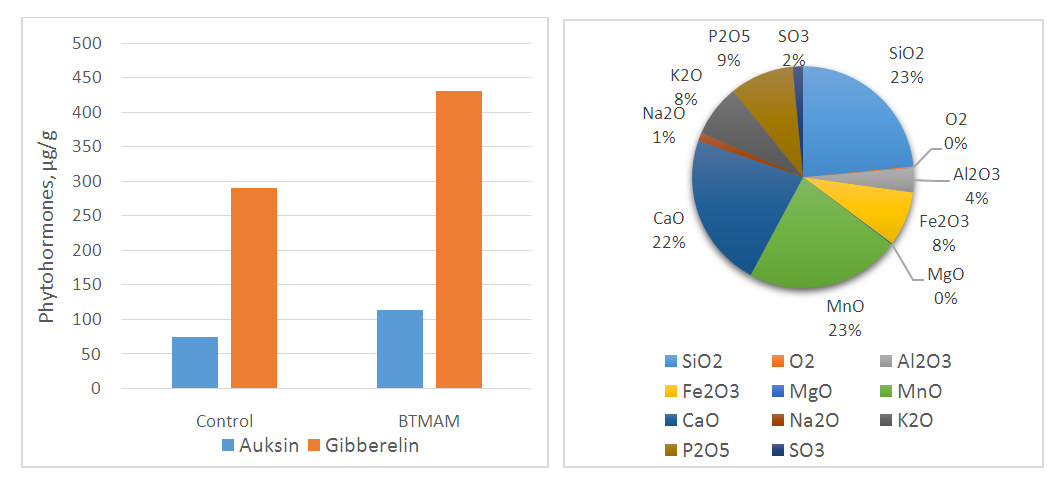 | Figure 3. (A) Content of phytohormones in BTMAM, (B) Mineral composition of BTMAM |
|
|
Author Contributions
- All authors contributed to the article and approved the submitted version.
Funding
- The study was carried out on the state assignment of the Academy of Sciences of the Republic of Uzbekistan, at the Institute of Microbiology.
ACKNOWLEDGEMENTS
- The work was carried out at the Institute of Microbiology Academy of Sciences of Uzbekistan (https://microbio.uz/).
Conflict of Interest
- The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML