-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Virology and Molecular Biology
p-ISSN: 2163-2219 e-ISSN: 2163-2227
2022; 11(4): 55-58
doi:10.5923/j.ijvmb.20221104.03
Received: Nov. 12, 2022; Accepted: Dec. 6, 2022; Published: Dec. 14, 2022

Analysis of Peripheral Immune Cells in the Post-COVID-19
Bakridin Zaripov, Gulsara Akhmedova
National University of Uzbekistan named after Mirzo Ulugbek, Tashkent, Uzbekistan
Correspondence to: Gulsara Akhmedova, National University of Uzbekistan named after Mirzo Ulugbek, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The given scientific article analyses the dynamics of quantitative alterations in peripheral leukocytes and hematopoietic elements in blood during the period of COVID-19 and recovery. We studied the effect of SARS-CoV-2 infection on the hematopoietic system, lymphocytopenia and eosinopenia during the course of the disease, control of the recovery period from the disease by monitoring the dynamics of the number of formed elements.
Keywords: COVID-19, Post-COVID-19, Physiology, Peripheral immune cells, Blood, Leukocytes, Lymphocytes, Monocytes, Neutrophils, Eosinophils
Cite this paper: Bakridin Zaripov, Gulsara Akhmedova, Analysis of Peripheral Immune Cells in the Post-COVID-19, International Journal of Virology and Molecular Biology, Vol. 11 No. 4, 2022, pp. 55-58. doi: 10.5923/j.ijvmb.20221104.03.
1. Introduction
- COVID-19 has become a global health threat. Hematological alterations in patients with Covid-19 disease may be one of the indicators of functional recovery, along with the immune response during SARS-CoV-2 infection [1,2,16]. Therefore, quantitative alterations in peripheral leukocytes as well as hematopoietic elements are currently being studied to determine whether COVID-19 is associated with fatal outcomes as an early signal in patients [3,4].Analyzing the scientific literature, the most common haematological symptoms in COVID-19 are a decrease in the number of lymphocytes - lymphocytopenia [5,6], an increase in the number of neutrophils - neutrophilia [7,8], as well as a decrease in the number of eosinophils - eosinopenia [9] and mild thrombocytopenia (35%) [10]. In many ways, eosinophils have been considered antiviral cells[11]. Eosinophils contain and produce molecules with antiviral activity and, thus, are involved in adaptive immunity, the properties of which have been studied in vitro and in vivo against a number of respiratory viruses, including influenza [12]. Of note, lymphopenia and eosinopenia were observed in 73 (52.9%) of 138 hospitalized patients with COVID-19. There is a positive correlation between lymphopenia and thrombocytopenia in patients with severe disease (r = 0.486, P <0.001) [13]. Therefore, in this article, a dynamic analysis of haematological indications for the treatment of COVID-19 in the postoperative period has been performed.
2. Materials and Methods
- General and biochemical analysis of blood was conducted mainly in the 16th family clinic of the Almazar district of Tashkent, in the multidisciplinary clinic of the Tashkent Medical Academy. Analyzes were performed on a biochemical analyzer BA-88A Mindray Co.Ltd (KNR). HUMAN (GmbH) reagents (Germany) were used. The study was conducted in a room with a moderate temperature (26°C) [16].The participation of study participants was voluntary and not funded. The study was conducted in accordance with the rules of scientific ethics, while maintaining the anonymity of the participants [15]. Cytometric studies were carried out according to the standards set on a BM 1800 microscope. Leukocyte counts were performed on a BM 1800 biological microscope using a Goryaev camera and automatic counters. Blood was taken under sterile conditions, for counting leukocytes 0.4 ml of a 3-5% solution of acetic acid was added to the solution stained with methylene blue. 20 µl of fresh blood (diluted 20 times) was taken with a capillary pipette and leukocytes were counted. Platelets were also counted according to the standards set in the Goryaev chamber under a BM 1800 biological microscope. The results were processed using the Excel and OriginPro6. 2017 programs (OriginLab Corporation, USA). The results were processed using Fisher's test, Student's t-factor. The arithmetic mean (M), mean deviation (±m), and statistical significance index (р) were determined. At р≤0.05, the results were considered 95% statistically significant.The research participants were divided into 4 experimental and 2 control groups in the post-COVID-19 period. For the 1st group, on the basis of voluntary consent, individuals with a severe course of COVID-19, under the age of 40 years, without chronic diseases were selected (n = 25). The 2nd group, on the basis of voluntary consent, were selected with moderate and mild forms of COVID-19, 2-3 months after recovery, not older than 40 years and without chronic diseases (n = 25). The second control group consisted of healthy people aged 41-55 years who were not infected with COVID-19 (n=12). The 3rd group, on the basis of voluntary consent, were selected with a severe course of COVID-19, 2-3 months after recovery, no older than 41-55 years, without chronic diseases (n=25). The 4th group, on the basis of voluntary consent, were selected with moderate and mild forms of COVID-19, 2-3 months after recovery, not older than 41-55 years, without chronic diseases (n = 25).
3. Results and Discussion
- For the study, people without concomitant chronic diseases who recovered from COVID-19 were selected. Functional parameters during the period of COVID-19 were obtained by retrospective analysis of case histories. Were obtained the results presented in fig. 1. for postoperative functional and haematological parameters.In the group of 30-40-year-olds, whose average age is 36.64±2.13 years, a retrospective analysis showed that the number of leukocytes in the disease was 6.68±0.37*10^9 cells/L in severe cases, 6.47±0.41*10^9cells/L in the lungs , which is somewhat lower than in the control norm, leukocytopenia was observed (P<0.05). On the 4th week after the disease it was 9.01±1.22*10^9cells/L in the first group, 8.79±0.41*10^9cells/L in the second group (P<0.05). It was found that leukocytes increased by 26% in both groups after recovery.In the second large group, the average age of the subjects was 46.1±1.87 years; as a result of retrospective and laboratory analysis, it was found that the number of leukocytes in the disease was 5.78±0.65 (P<0.05) in severe cases, and in non-severe cases - 7.08 ± 0.81 (P<0.05), mild leukocytopenia was observed somewhat less than the control norm (P<0.05), at the 4th week after the disease - 8.75 ± 1.78 in the 3rd group, 9.15 ± 0, in the fourth group - 68 (P<0.05). Leukocytes increased by 19.2% in the first group and by 19.7% in the second group after recovery (Fig. 1).
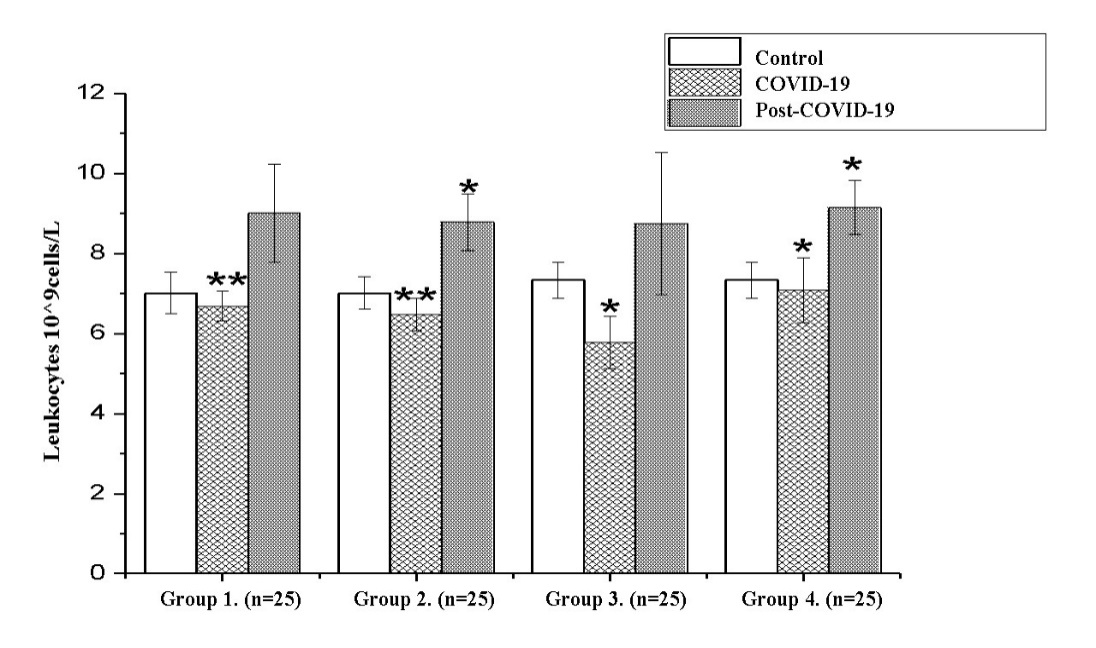 | Figure 1. Analysis of leukocytes during the recovery period (M±m) (*- Р<0,05; **-Р<0,01; ***-Р<0,001) |
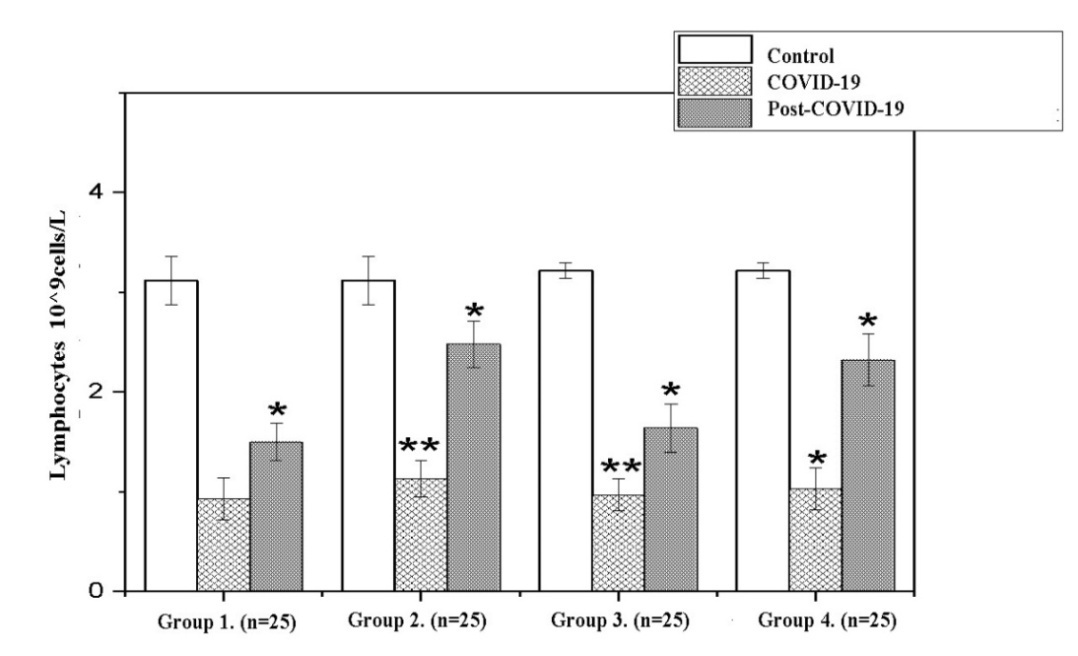 | Figure 2. Dynamics lymphocytes in the recovery period (Note: Unit of measurement - 10^9cells/L; *- P<0,05; **-P<0,01; ***-P<0,001) |
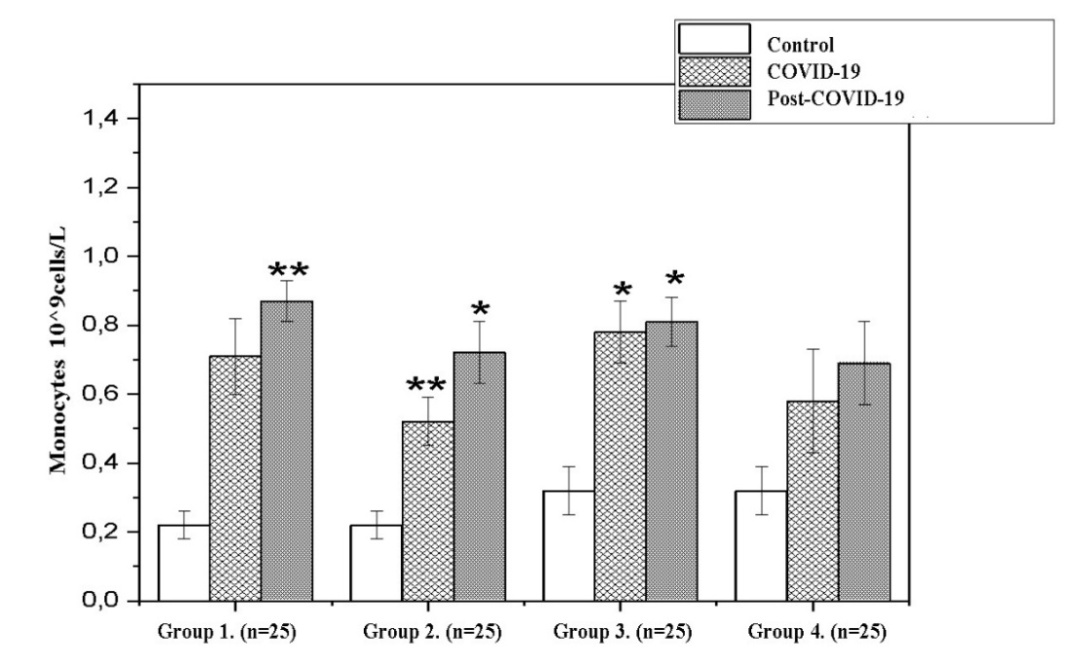 | Figure 3. Dynamics monocytes in the recovery period (Note: Unit of measurement - 10^9cells/L; *- P<0,05; **-P<0,01; ***-P<0,001) |
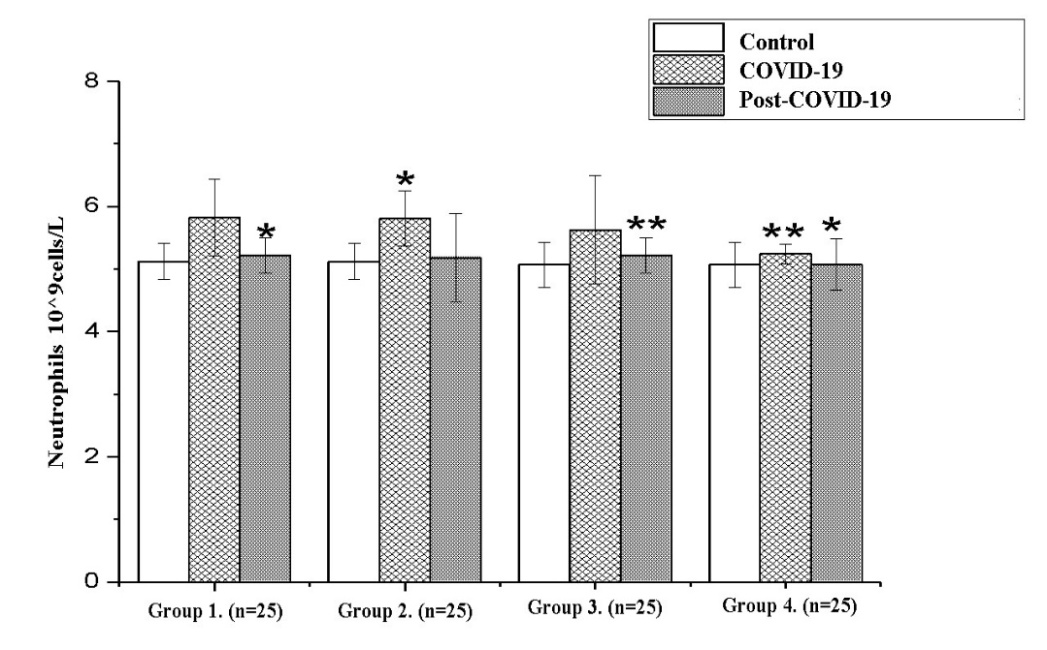 | Figure 4. Dynamics neutrophils in the recovery period (Note: Unit of measurement - 10^9cells/L; *- P<0,05; **-P<0,01; ***-P<0,001) |
4. Conclusions
- Thus, COVID-19 is a systemic disease that significantly affects the haematopoietic system and haemostasis. Since lymphocytopenia and eosinopenia are observed during the course of the disease, dynamic control of the number of hematopoietic elements is one of the most important factors in recovery. As a result, monitoring of haematological parameters, the dynamics of the number of lymphocytes helps to control the period of recovery from the disease and take timely preventive measures.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML