-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Virology and Molecular Biology
p-ISSN: 2163-2219 e-ISSN: 2163-2227
2022; 11(4): 50-54
doi:10.5923/j.ijvmb.20221104.02
Received: Oct. 6, 2022; Accepted: Oct. 28, 2022; Published: Oct. 31, 2022

Study of the Anti-Diabetes Activity of Polyphenols Contained in Plantago Major L. Plant
F. Sh. Tokhtaeva1, R. R. Makhmudov2, N. M. Yuldashev3
1Doctoral Student, Chirchik State Pedagogical University, Uzbekistan
2Senior Research Fellow, Doctor of Philosophy in Chemistry, Institute of Bioorganic Chemistry Named after Academician O.S. Sodikov of the Academy of Sciences of the Republic of Uzbekistan
3Doctor of Biological Sciences, Tashkent Pediatric Medical Institute, Uzbekistan
Correspondence to: F. Sh. Tokhtaeva, Doctoral Student, Chirchik State Pedagogical University, Uzbekistan.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Polyphenolic compounds interact with free radicals and slow down lipid oxidation in the body due to the formation of inactive phenol radicals. In type 2 diabetes mellitus, the hypoglycemic effect of polyphenols occurs through several mechanisms at once: inhibition of the breakdown and absorption of complex carbohydrates in the intestine, protection of pancreatic β–cells from destruction and stimulation of their secretory activity, activation of peripheral tissue insulin receptors, and control of glucose release from the liver. Many studies in experimental animals and cell culture indicate that polyphenols have a positive effect on β–cell function.
Keywords: Plantaginaceae, Plantago major L, Plantago lanceolata L. diabetes, Glucose, Cholesterol
Cite this paper: F. Sh. Tokhtaeva, R. R. Makhmudov, N. M. Yuldashev, Study of the Anti-Diabetes Activity of Polyphenols Contained in Plantago Major L. Plant, International Journal of Virology and Molecular Biology, Vol. 11 No. 4, 2022, pp. 50-54. doi: 10.5923/j.ijvmb.20221104.02.
1. Introduction
- According to the data provided by the World Health Organization, diabetes is the first among the diseases that cause disability, among the diseases that cause death, arteriosclerosis takes the third place after cancer. Experts around the world consider diabetes as a non–infectious, but rapidly spreading disease, like cardiovascular and oncological diseases. Diabetes mellitus is a disease that has become widespread in the world in recent years, and 1–8% of the world’s population suffers from this disease. According to the results of the investigation, the number of patients increases more than twice every 10 years. Today, more than 422 million people are infected with this disease, and the number of people who die from complications of this disease is 5 million per year consists of more than that is, every 6 seconds, 1 person in the world dies from complications of diabetes. 10 per year and more than a million patients remain disabled. In Uzbekistan, this figure is over 130,000. Unfortunately, the number of people suffering from this disease is increasing year by year and is getting younger and younger. Glucose contained in food products consumed by humans is the main source of energy for all organs and tissues. The brain's daily consumption of glucose is 115 g, that is, 75–100 mg per minute. Glucose that enters the blood is quickly broken down into its components and provides the body with quality energy, the remaining part is stored in the form of glycogen in the liver and tissues, and the remaining part is transferred to the form of lipids for storage.Diabetes is one of the endocrine diseases, as a result of a complete or partial deficiency of the insulin hormone, hyperglycemia is characterized by a continuous increase in the amount of glucose in the blood, a violation of metabolic processes in the body (carbohydrate, fat, protein, mineral and water–salt metabolism) and irreversible organic is characterized as a chronic disease causing pathologies.Finding new effective methods of diabetes treatment is one of the main problems of the world medicine and health organization. The negative impact of the external environment, population specificity, a number of risk factors (overweight, arterial hypertension, vascular diseases, hyperlipidemia, etc.) cause the wide spread of this disease. Medical science is making significant progress in prolonging the life of patients with diabetes and reducing the complications of the disease. In diabetes, for therapeutic purposes, sugar–lowering agents and insulin are mainly used. However, when xenobiotics enter the body, activation of the microsomal oxidation system occurs, the formation of free radicals increases, and this leads to cell and tissue damage.Therefore, recently there has been a growing interest in natural substances, including compounds extracted from plants. Phenolic compounds are widely distributed in the plant world and are the main products of plant metabolism. Phenolic compounds are well known to possess a wide spectrum of biological activities, including antioxidant, membrane, and immune–stimulating properties. Currently, there is information that plant polyphenols influence the indicators of carbohydrate metabolism and the activity of the insulin–producing organ, and that some phenolic compounds normalize the function of blood vessels. However, the biochemical basis of these effects is poorly understood [1].Among the biologically active substances that regulate the activity of blood vessels, NO is of particular importance, and the production of NO is impaired in diabetes. Therefore, in order to understand the mechanism of complications caused by diabetes mellitus in blood vessels, it is necessary to consider in detail the biochemical role of nitric oxide in the body.Insulin is synthesized in β–cells of the pancreas, and nitric oxide (NO) is of great importance in the destruction of these cells. It has the ability to induce relaxation of blood vessels, alter the transmission of nerve signals and respond to phagocyte toxicity. Nitrous oxide (NO) is also formed during the conversion of L–arginine to L–citrulline under the action of NO–synthase enzyme. Due to the activation of free radical oxidation processes in type 1 diabetes mellitus, the amount of NO–synthase enzyme in endothelial cells decreases compared to its substrate – arginine. Decreased production of NO leads to narrowing of blood vessels and impaired circulation. In type 2 diabetes, the increased production of NO is caused by a large amount of the toxic compound ONOO – leads to the formation of As a result, it damages blood vessels and causes atherosclerosis.Antioxidants are among the mandatory components of the complex treatment of diabetes, and it is urgent to study the effect of natural antioxidants extracted from plants on the peroxidation of lipids and the progress of microangiopathy in patients with diabetes. Flavonoids and their derivatives are considered heterocyclic compounds, which exhibit the properties of reducing the permeability of blood vessels and the fragility of their walls due to their antioxidant and membrane stabilization properties. Polyphenolic compounds interact with free radicals and slow down lipid oxidation in the body due to the formation of inactive phenol radicals.Polyphenols are biologically active components of many plants and have been used in folk and traditional medicine since ancient times. More than 8,000 compounds with a polyphenolic structure have been identified in plants. They can be found in plants in the form of simple monophenolic acid (hydroxybenzoic or hydroxycinnamic acid), as well as compounds with several aromatic rings, which are very common in nature–flavonoids. The flavonoid molecule consists of two aromatic rings connected by three carbon atoms, which together with an oxygen atom form a central pyran heterocycle. According to the degree of oxidation of the pyran ring, flavonoids are divided into different classes – anthocyanins, flavonols, flavans, flavanols, flavonones. In plants, flavonoids are mainly glycosidated and in the form of other conjugates, oligo– and polymer derivatives – proanthocyanidins and condensed tannins can also be formed. Non–flavonoid polyphenols include hydrolyzable tannins, stilbenoids (resveratrol) and lignans [2,3].For many years, the biological activities of polyphenols have been linked to their antioxidant properties. To date, it has been confirmed that natural polyphenols have a direct interaction with proteins (peptides) as the basis of the mechanism of preventing the development and proliferation of chronic diseases, including diabetes. The formation of polyphenol–protein complexes can be explained by the hydrophobic attraction of the polyphenol aromatic ring and the non–polar radical of the protein amino acid, resulting in the formation of hydrogen bonds between the phenol group of polyphenols and the carbonyl group of proteins. As a result of this interaction, depending on the target protein, the activity of the enzyme system, cell receptor changes [3].In type 2 diabetes mellitus, the hypoglycemic effect of polyphenols occurs through several mechanisms at once: inhibition of the breakdown and absorption of complex carbohydrates in the intestine, protection of pancreatic β–cells from destruction and stimulation of their secretory activity, activation of peripheral tissue insulin receptors, and control of glucose release from the liver.Among phenolic acids, caffeic, chlorogenic and tannic acids are distinguished by their high inhibitory activity. Numerous studies in experimental animals and cell culture indicate that polyphenols have a positive effect on β–cell function. In addition, due to their antioxidant properties, polyphenolic compounds (apigenin, quercetin, luteolin, rutin, etc.) protect β–cells from harmful endogenous factors (active forms of oxygen, nitric oxide, cytokines). Glucose transport in muscle and adipose tissue cells is controlled by insulin. In the experiments, there was an increase in glucose transport under the influence of chlorogenic and ferulic acids, catechin, cyanidin–3–glucoside, some flavonones and flavonols, and resveratrol [2,4,5,6].The liver plays a key role in maintaining the constant level of glucose in the blood, and it controls the process of storing and releasing glucose. In type 2 diabetes, these processes go out of control, resulting in hyperglycemia. Polyphenols can regulate glucose excretion by directly influencing the activity of enzymes in the liver.Phytopreparations are usually used in complex therapy in the treatment of patients with diabetes in the early stages of the disease. They have an advantage over synthetic agents due to their low toxicity and long–term administration without side effects, wide range of pharmacological effects, and low cost.Nitric oxide (NO) is formed from the amino acid L–arginine, which is catalyzed by the NO synthase enzyme. As a reaction product, NO and L–citrulline amino acid are formed. As a result of the effect of nitric oxide on oxygen in the blood, stable products–nitrite and nitrate are formed, which are markers that determine the amount of nitric oxide in the body. In type 1 diabetes mellitus, the amount of NO–synthesis in endothelial cells decreases compared to its substrate L–arginine, which stimulates the activation of free radical reactions. A decrease in the amount of nitric oxide leads to narrowing of blood vessels and impaired circulation. In type 2 diabetes, on the contrary, due to the increase in nitric oxide, there is an increase in the amount of nitrates in the blood, and a decrease in arginine, which leads to an increase in the formation of the toxic compound ONOO–. As a result, blood vessels are damaged and atherosclerosis occurs. Under the influence of polyphenols, the amount of nitrates, arginine and citrulline is normalized in both types of diabetes.Carbohydrates in food, including polysaccharides (starch), disaccharides (lactose, sucrose) are exogenous sources of glycemia. After entering the body, complex carbohydrates are broken down into monosaccharides due to enzymatic breakdown. Glucose, the last major product of carbohydrate breakdown, is quickly absorbed and added to the bloodstream. α–amylase, α–glucosidase are key enzymes involved in the breakdown of complex carbohydrates, and they are secreted from the pancreas into the intestines. Inhibition of these enzymes under the influence of various antidiabetic drugs slows down the absorption of glucose into the blood, facilitates the function of the pancreas and protects it, and also reduces insulin resistance [11].Many polyphenols have the property of inhibiting α–amylase and α–glucosidase. The activity of certain polyphenols (myricetin, luteolin, luteolin–7–glucoside) can be compared with the drug (acarbose) used in clinical medicine [10]. A synergistic effect was achieved with the combination of acarbose with epigallocatechin gallate in small doses [11].When studying the inhibitory activities of polyphenolic compounds, it was found that their inhibitory effect depends on the number of free hydroxyl groups, their location and the length of the hydrocarbon chain in the polyphenol molecule. For example, among phenolic acids, caffeic, chlorogenic, and tannic acids, which contain several adjacent hydroxyl groups in the benzene ring, have a high inhibitory activity toward birman. In turn, ferulic acid (the ON–group is methoxylated) and gallic acid (with a short molecular chain) have a weak inhibitory effect showed [12].Glucose absorption in the intestine is carried out in different ways, and at very low concentrations of glucose, enterocytes absorb it against the concentration gradient by active transport with the participation of the sodium–dependent transport protein SGLUT1. At a high concentration of glucose, its diffusion into the cell is facilitated with the help of the membrane protein transporter GLUN2 [13].In vitro as a result of research, flavonoid and phenolic acids have been found to inhibit glucose absorption.Insulin secretion is controlled by glucose, which enters the β–cells of the pancreas through the bloodstream. In β–cells, glucose is enzymatically transformed into adenosine triphosphate (ATF). Intracellular accumulation of ATF leads to the closing of potassium ion channels, depolarization of the cell membrane, and the opening of calcium channels. The influx of calcium ions into the cells causes insulin stored in secretory granules to be released into the intercellular space, and then into the blood [14].Prolonged hyperglycemia in type 2 diabetes leads to impaired β–cell function, decreased insulin secretion, and eventually β–cell apoptosis resulting in decreased cell weight [15].Many studies in experimental animals and cell culture indicate that polyphenols have a positive effect on β–cell function. It has been found that polyphenols can affect the chain of biochemical reactions associated with the release of insulin. In particular, an increase in insulin secretion by catechin–gallates was observed with inhibition of the activity of ATF–sensitive potassium channels, and an increase in the amount of potassium ions was observed in the presence of genestein. In addition, due to their antioxidant properties, polyphenolic compounds (apigenin, quercetin, luteolin, rutin) protect β–cells from endogenous damaging factors (active forms of oxygen, nitric oxide, cytokines) and prevent cell destruction [7,16].The metabolism of polyphenolic compounds begins in the cells of the intestinal mucosa and is carried out by means of glucuronidation and methylation reactions. From the enterocytes, polyphenols pass into the bloodstream and are transported through the veins to the liver, where their further metabolism is continued. [2,17,18].Under the influence of enzymes in the liver, the 1st phase of polyphenol metabolism undergoes oxidation, reduction, hydrolysis reactions and conjugation. Conjugation includes sulfation, methylation, and partial glucuronation reactions. Conjugation reactions take place faster than oxidation reactions and are characterized by high efficiency. The presence of native polyphenol aglycones in very small amounts or not at all in the blood plasma testifies to the high efficiency of this reaction. Intensive metabolism of polyphenols makes it difficult to determine their bioefficacy.Through the bloodstream, polyphenol metabolites are distributed to various organs and tissues. As a result of research conducted in experimental animals, polyphenol compounds are observed in brain tissue, endothelial cells, heart, kidney, spleen, pancreas, bladder and skin. Polyphenols are excreted through bile or kidneys. Larger conjugates are mainly excreted through the bile, while smaller conjugates, such as monosulfates, are excreted through the kidneys. Fast excretion is typical for anthocyanins and phenolic acids, while quercetin and its glycosides are excreted very slowly and may even accumulate [2,19].To the above informationbased on this, we set ourselves the goal of studying the chemical composition of P. major L. and P. lanceolata L. plants, which are rich in phenolic compounds and widely distributed in our Republic, in order to create effective drugs against diabetes.Based on the above information, in order to create effective drugs against diabetes, we set ourselves the goal of studying the biochemical activity of the polyphenolic substance extracted from P. major L. and P. major L. lacelota plants, which are rich in phenolic compounds and widely distributed in our Republic, in experimental diabetes.
2. Work Progress
- The study was conducted on white laboratory rats that were quarantined for 14 days. 84 white male healthy rats with a body weight of 145.0–250.0 grams were selected for the experiment. Rats were divided into 6 to 7 groups (intact, control, experiment+diabetes, 50 mg Pl.m (50mg/kg of Plantago major substance included), 100 mg Pl.m (100mg/kg of Plantago major substance included) and 50 mg Pl.L (Included 50mg/kg of Plantago lancelota substance), 100mg Pl.L (Included 100mg/kg of Plantago lancelota Plantago major substance)) were separated and identified as the experimental group.
3. Method
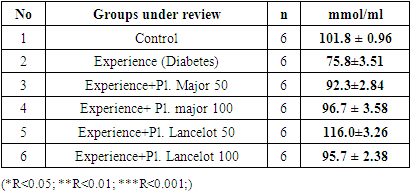
- In rats, symptoms of type 1 diabetes were induced by intraperitoneal injection of Alloxan at a dose of 150 mg/kg, and symptoms of type 2 diabetes were produced by a single dose of streptozocin at 35 mg/kg [20].Before starting the experiment, blood was taken under diethyl ether anesthesia for glucose, insulin, glycated hemoglobin levels and biochemical tests, and these results were taken as a control. 7 days after alloxan and streptozocin administration, the glucose level was re–checked, and for the treatment, Plantago major at doses of 50 and 100 mg/kg and the sum of polyphenols extracted from Plantago lancelota plant at doses of 50 and 100 mg/kg, and the control group animals were given an equal volume of distilled water. The amount of glucose after 7, 14 and 21 days was checked by inserting a special probe into the stomach. The obtained results were compared with the control group. Blood tests were performed on the HUMAN STAR–100 machine.
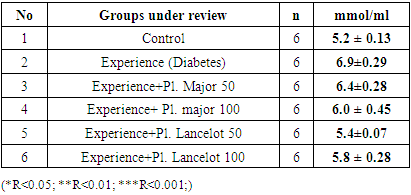
4. Results
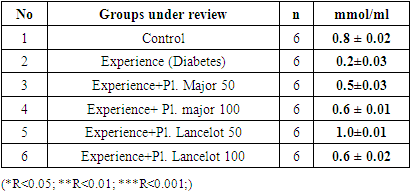
|
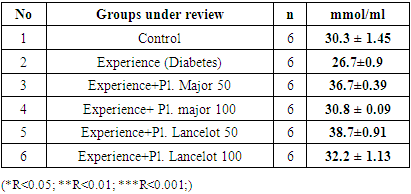
|
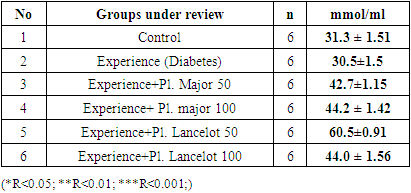
|
|
|
5. Summary
- In the experiment, we studied the changes of some biochemical indicators (glucose, cholesterol, creatinine, alanine aminotransferase, aspartate aminotransferase) in the body of white male rats. The obtained results were studied in comparison with the control group. In the experiment, we saw that the substance of Plantogo lancelota L. 50 mg/kg has an active effect on biochemical parameters.Due to the presence of a phenol group in the composition, polyphenols affect certain protein targets in various organs and tissues (small intestine, pancreas, liver, peripheral tissues) and show the property of controlling the amount of glucose in the blood. The hypoglycemic effect of polyphenols in type 2 diabetes mellitus has been confirmed in experiments by several mechanisms, including the breakdown of complex carbohydrates and inhibition of glucose absorption in the intestine, protection of pancreatic β–cells from destruction and stimulation of their secretory activity, activation of insulin receptors in peripheral tissues, and can be cited as controlling the release of glucose from the liver. Many polyphenols have the property of inhibiting enzymes involved in the breakdown of glucose. In this case, the inhibitory effect of some compounds (myricetin, luteolin, luteolin–7–glucoside) can be equated with the effect of drugs.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML