R. K. Gulomov1, A. R. Batoshov2
1Doctoral Student, Namangan State University, Namangan, Uzbekistan
2Doctor of Biological Sciences, Associate Professor, Namangan State University, Namangan, Uzbekistan
Correspondence to: R. K. Gulomov, Doctoral Student, Namangan State University, Namangan, Uzbekistan.
| Email: |  |
Copyright © 2022 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
This article presents 22 morphological characters of the genus Phlomoides distributed in the Fergana Valley and the primary and progressive signs of evolution of root, leaf, calyx and crown color as the main taxa, their diagnostic analysis and phylogenetic tree. Phylogenetic analyzes were performed on the data matrix using the PAUP* v4.0b10 program using the maximal parsimonious method (MP). The results obtained and morphological characters are integrated into Mesquite v3.02.
Keywords:
Fergana Valley, Phlomoides, Phylogeny, Morphology, PAUP
Cite this paper: R. K. Gulomov, A. R. Batoshov, Morphological Phylogeny of the Species Phlomoides Moench (Lamiaceae) Distributed in the Fergana Valley, International Journal of Virology and Molecular Biology, Vol. 11 No. 1, 2022, pp. 9-15. doi: 10.5923/j.ijvmb.20221101.03.
1. Introduction
In recent years, a number of studies on molecular (Salmaki et al., 2012a, 2012b), morphological (Eyvazadeh and Salmaki, 2019) and seed micromorphology (Eyvazadeh and Salmaki, 2018), cytogenetic (Ranjbar et al., 2016) and taxonomy of the Phlomoides genus was carried out. These studies have been conducted in a regional and narrow framework and do not provide definitive comprehensive data on the genus. To date, many taxonomic studies have been conducted on a number of species (Adylov et al., 1986; Kamelin and Maxmedov, 1990; Salmaki et al., 2012b; Sennikov and Lazkov, 2013) but none of them is complete. Based on the above data, there is a need to conduct extensive taxonomic, geographical, ecological and phylogenetic research on the Phlomoides series in close collaboration with scientists from Iran, Central Asia and China.We aim to shed light on the morphological phylogeny and their diagnostic analysis based on 22 morpho – characters of the genus species distributed in the Fergana Valley. This is the first attempt in Central Asia to determine the evolutionary morpho – characters of a species based on modern methods. We hope that our phylogenetic analyzes based on the morphological characteristics of the species of Phlomoides will serve in some way in revealing the place of the morphological features in the origin of the genus.
2. The Main Results and Findings
Study area and plant materialThe Fergana Valley covers less than 1% of Central Asia (22,000 km2). It is 300 km long (east to west) and 80 – 100 km wide (north to south). The height of the valley is 3300 m in the eastern part of Kyrgyzstan and 1050 m in the western part (Khojand) (Kaparkar, 2019). The valley is surrounded by ridges that are part of the Western Tien Shan and Gissar – Alay mountain systems. It is surrounded by Qurama in the northwest, Chatkal in the north, Fergana and Otoynak in the northeast and east, Alay and Turkestan in the south and Mogultog in the west (National Encyclopedia of Uzbekistan, 2005). The mountains descend to the center of the valley and merge with the plains. The valley is politically and administratively located in the territory of three countries – Uzbekistan, Kyrgyzstan and Tajikistan. (Fig. 1).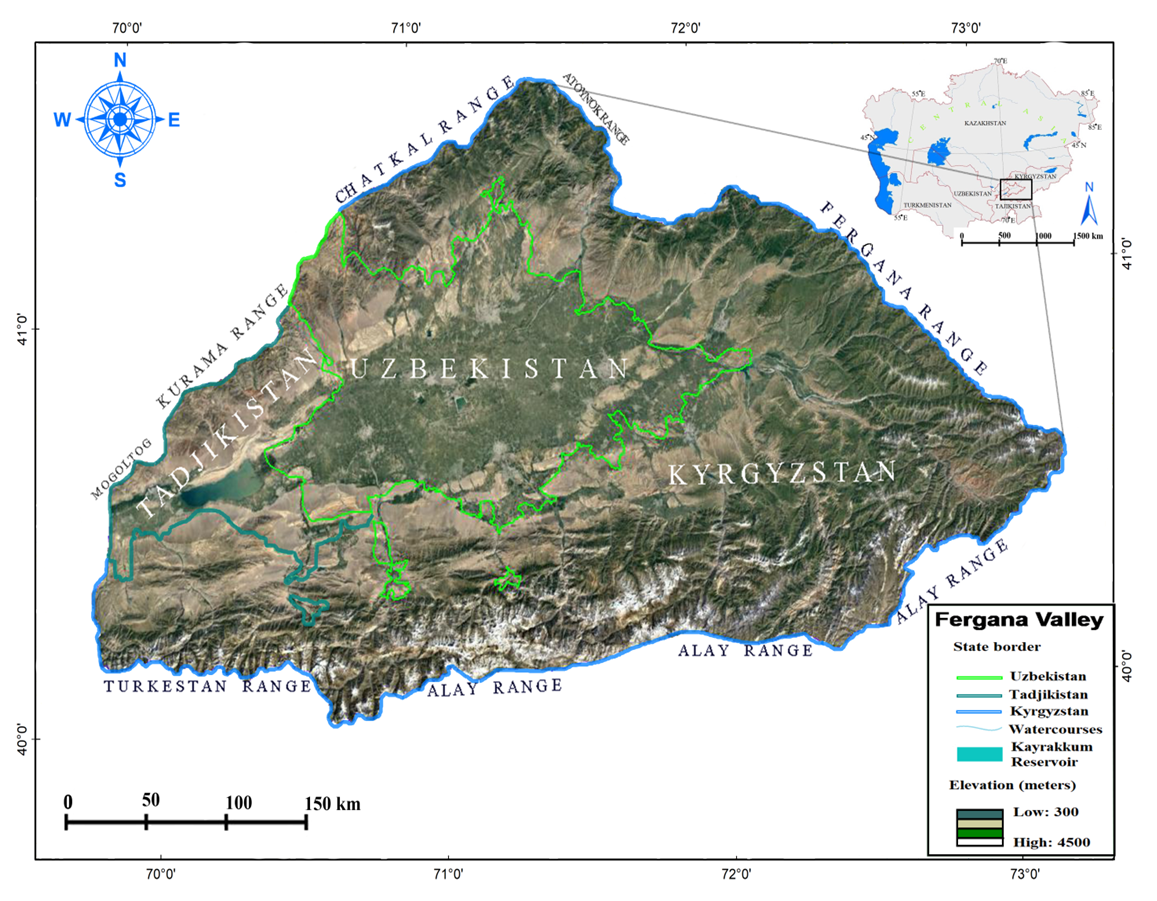 | Figure 1. General topography of the Ferghana Valley in Central Asia |
Morphological evaluationOur study was covered following a taxonomic classification published by Sennikov and Lazkov (2013). The classification recognizes 5 sections of the Phlomoides genus. 26 species of the genus Phlomoides and 22 morphological features of one outer group (Phlomis hypoleuca) were studied. The analyzed morpho – characters are represented by numbers from two to four (Table 1). Morphological features of species National Herbarium of Uzbekistan (TASH), Based on the analysis of samples collected in targeted field research for 2021-2022 and the botanical classification mentioned in the Lazkov monograph (2016). Primary and progressive morpho – characters of the species were described based on Makhmedov (1991) classification.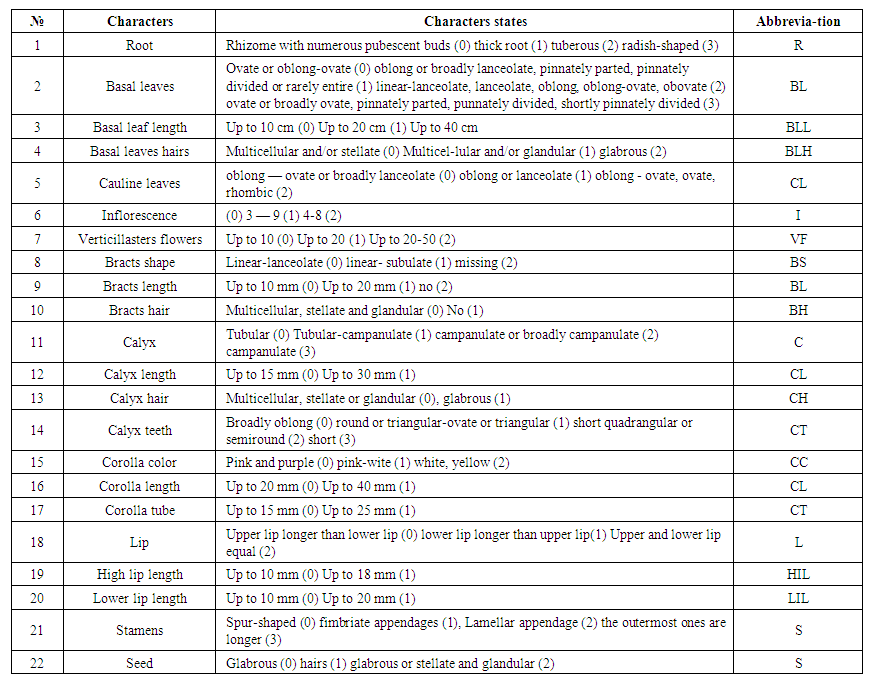 | Table 1. List of morphological features of species of Phlomoides and their abbreviations |
Phylogenetic analysisPhylogenetic analyzes were performed on the data matrix (Table 2) using the PAUP* v4.0b10 (Swofford 2002) program using the maximal parsimonious method (MP). All characters were considered to have the same weight. The heuristic search option was selected using 100 replications of a random addition sequence with ACCTRAN optimization and replacement of TBR (splitting tree) branches. The reconnection limit is set to 8. We then applied a sequential retraction strategy to improve tree indices and reduce the effect of characters representing homoplasia on tree topologies (Farris 1969). Using equal weight symbols, the length of the first phylogenetic tree was determined to be 1.0 the consistency index CI = 0.462 the retention index RI = 0.731 and RC emained unchanged in successive rounds, these trees were accepted as the successive re-weighting trees. 100 trees were taken. In all analyzes, supports were revalued using the heuristic search option, simple addition sequence, TBR branch replacement and 10000 replications without MulTrees (Felsenstein 1985) and the maximum parsimonious heuristic search option retained the best single tree (Fig. 2). The relationship between the species of Phlomoides has not been resolved in this tree. The results and morphobes obtained in the PAUP program were integrated into Mesquite v3.02 (Maddison and Maddison, 2015) and the relationship between the characters was shown (Fig. 3).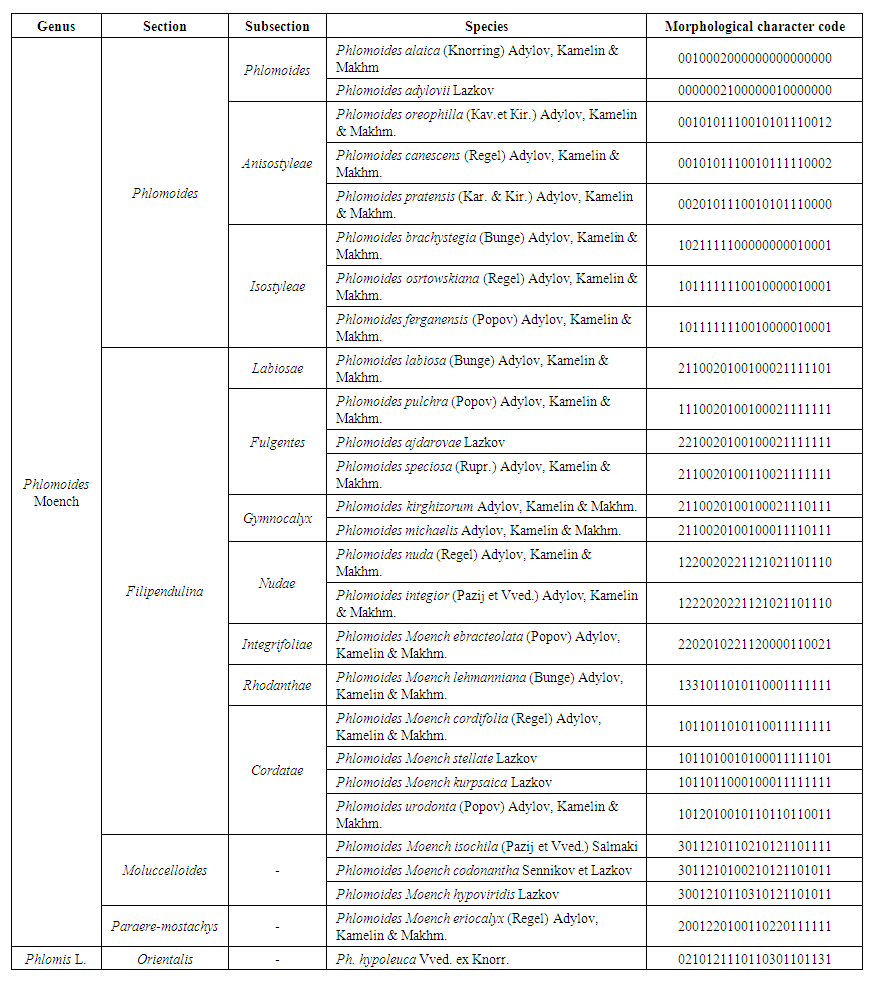 | Table 2. Data matrix used in phylogenetic analysis of Phlomoides Moench |
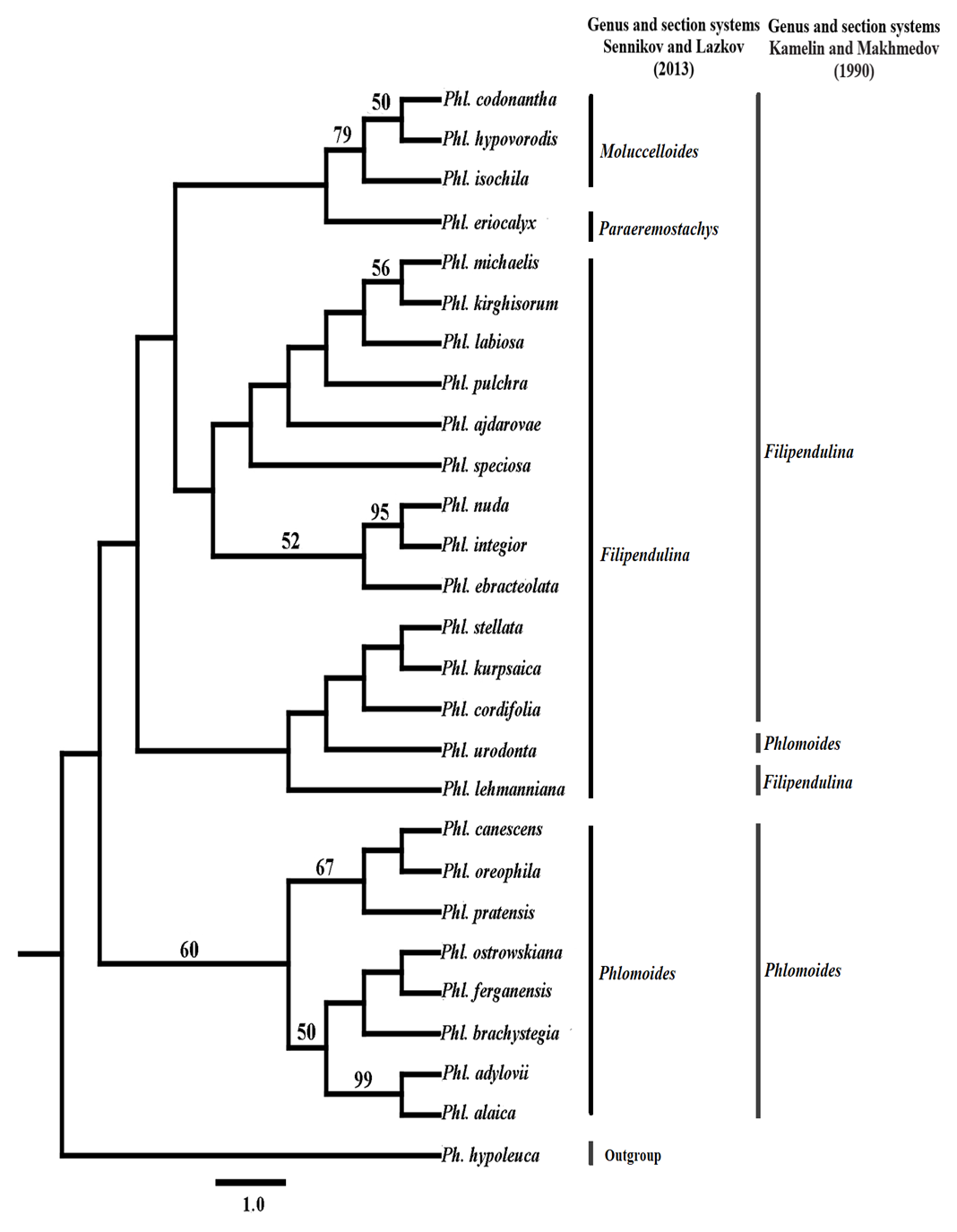 | Figure 2. Strict consensus tree derived from the morphological data matrix after successive weighting by rescaled consistency index and the correspondence of the species sectional positions to taxonomic classifications of Sennikov & Lazkov (2013) and Kamelin & Makhmedov (1990). Numbers above branches are bootstrap values. Numbers <50% are not shown |
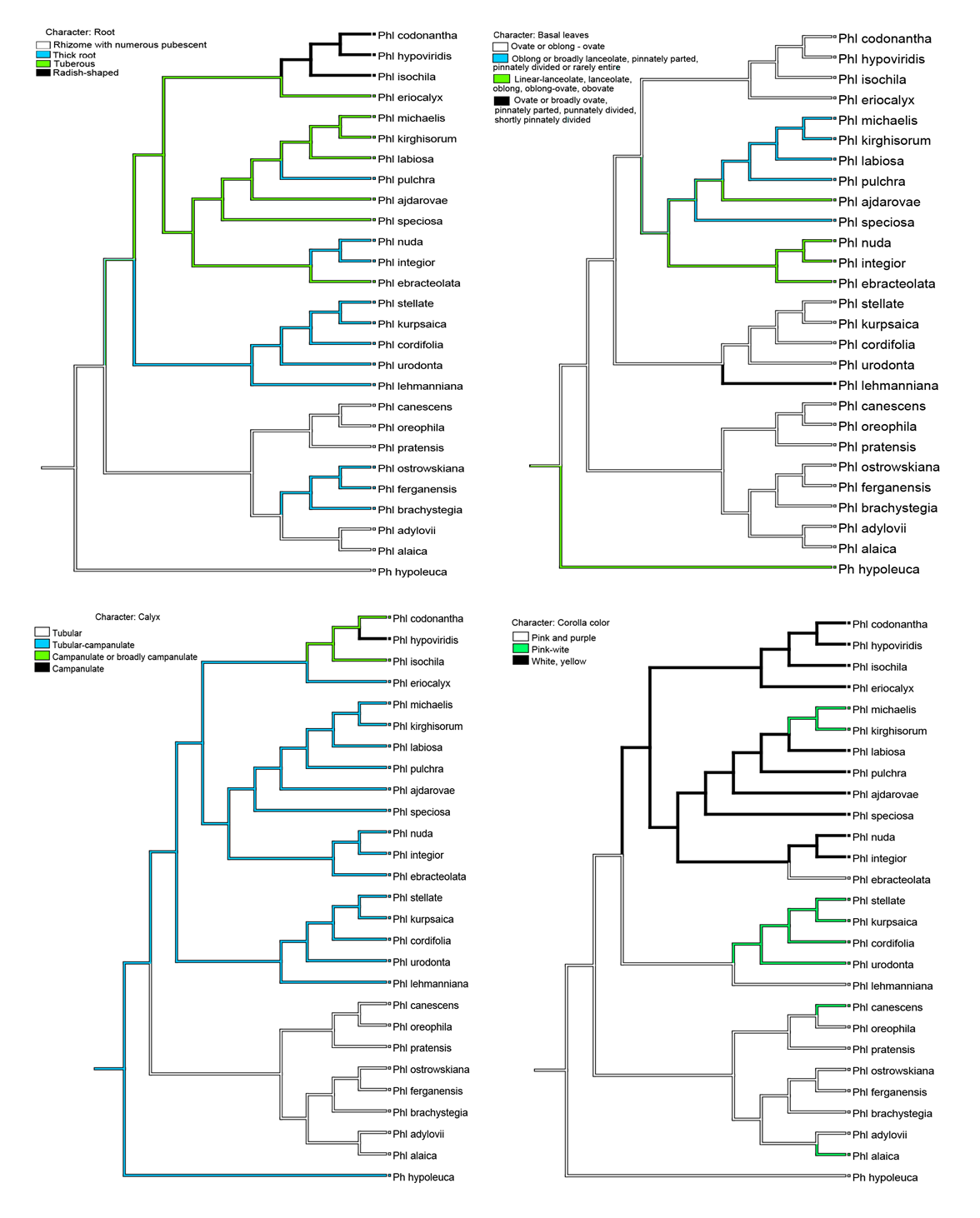 | Figure 3. Reconstruction of the evolution of four morphological characters in Phlomoides: Root, basal leaves, calyx and corolla color |
The essence of the morphological method is to divide the primary and progressive characters into two opposite groups. The evolution of characters determines the evolution of taxa. If the original changes of morpho-characters in the adopted taxon are preserved, they will have to be placed in the lowest place in the system. To do this, the characters are identified by codes, which are used to determine the relative height of the level of development and the position of the species in the taxonomy (Мakhmedov, 1991). Based on the phylogenetic basis published by Salmaki (2012a) they presented an updated system of the Phlomoides genus. Sennikov and Lazkov (2013) Phlomoides genus into 5 section that were monophyletic. Including: Phlomoides (Bunge) Sennikov, Eremostachys (Bunge) Sennikov, Filipendula Kamelin & Mahmedov, Moluccelloides (Bunge) Sennikov and Paraeremostachys (Adylov, Kamelin & Makhm) Sennikov. I Section Phlomoides (Bunge) Sennikov, Flora, 213 (2015) 40–48.The section of phlomoides differs from other sections morphologically and biogeographically. This section is best expressed in China, including Yunnan and Sichuan provinces. In our analysis, the section is represented by 8 species distributed in the valley. The leaves are simple, the leaf blade toothed or whole. The flowers are always the same color; purple, pink, yellow or white, upper lip arched.II Section Filipendulina Popov ex Kamelin & Machmedov in Bot. J. (Moscow & Leningrad) 75(2): 245 (1990).The Filipendula section represents the largest and most diverse and controversial group in the Phlomoides genus. In our analysis, the section is represented by 14 species distributed in the valley. The leaves are pinnate (rarely - rarely the whole), the inflorescence is two different colors.III Section Molucelloides (Bunge) Sennikov, 2013, Memoranda Soc. Fauna Fl. Fenn. 89: 132. From a phylogenetic point of view, the representatives of this section are monophyletic (Salmaki et al., 2012a) and occupies a lower link compared to the other species in the genus.In our analysis, the section is represented by 3 species distributed in the valley. The main root is turnip, the leaves are whole, the leaf blade is finely toothed or blunt. The calyx is funnel-shaped, the inflorescence is mostly yellow.IV Section Paraeremostachys (Adylov, Kamelin & Machmedov) Sennikov, 2013, Memoranda Soc. Fauna Flora Fennica, 89: 133.The results of molecular phylogenetic studies conducted on the genus Phlomideae (Salmaki et al., 2012a) suggest that there is no basis for recognizing Paraeremostachys as a separate tribe. Thus, Sennikov and Lazkov (2013) attached the level of this title to a section of the Phlomoides genus. In our analysis, the section is represented by 1 species distributed in the valley (calyx bell-shaped). The discussion of the article cites the role of morpho-characters in the phylogenetic tree based on the structure of the root, leaf, calyx and corolla as the main taxonomic characters. The evolutionary role and significance of the remaining taxonomic characters, as well as comments on the genesis of the origin of the genus proposed by Popov (1940) and Makhmedov (1991) are not discussed in this article.The evolutionary role of taxonomic traits in morphological phylogeny (Fig. 3) is given and the primary and progressive morpho signs are given on the basis of Makhmedov (1991) classification. Including; Non-thickened main and additional roots - the main root is not thickened, additional roots are thickened in the form of one or 2-3 turnips - additional roots are not thickened. The whole leaf - patsimon carved or slightly trimmed - divided leafCalyx tubular - bell-shaped - funnel-shapedCorolla color monochrome - heterochrome
3. Conclusions
It can be said that the species of this series has undergone many morphological stratifications in its origin. On the one hand there are whole-leaved species prone to forest landscape, on the other hand there are species with cut or divided leaves adapted to xerophilous conditions. As a result of natural hybridization of the species distributed in the intersection areas, many intermediate forms were formed. This situation can be considered as a key factor in the evolution of polymorphisms of species.
References
| [1] | Salmaki, Y., Zarre, S., Heubl, G., 2012b. The genus Phlomoides Moench (Lamiaceae; Lamioideae; Phlomideae) in Iran: an updated synopsis. Iran. J. Bot. 18, 207–219. |
| [2] | Salmaki Y., Zarre S., Ryding O., Lindqvist C., Schneunert A., Brauchler C. & Heubl G. Phylogeny of the tribe Phlomideae (Lamioideae: Lamiaceae) with special focus on Eremostachys and Phlomoides: New insights from nuclear and chloroplast sequences // Taxon, 2012. 61 (1): – P. 161 - 179. |
| [3] | Seyedi Z. and Salmaki Y. 2015. Trichomes morphology and its signifiance in the systematics of Phlomoides (Lamiaceae; Lamioideae; Phlomideae). – Flora 213: 40–48. |
| [4] | Elmira E. and Salmaki Y. 2019. Evolution of trichome types and its systematic significance in the genus Phlomoides (Lamioideae–Lamiaceae). – Nordic Journal of Botany. 1-14. |
| [5] | Elmira E. and Salmaki Y. 2019. Evolution of trichome types and its systematic significance in the genus Phlomoides (Lamioideae–Lamiaceae). – Nordic Journal of Botany. 1-14. |
| [6] | Khosroshahi E.E. & Salmaki Y. 2018. Nutlet micromorphology and its systematic implications in Phlomoides Moench (Lamiaceae). Nova Biologica Reperta 5 (1): 82-94. |
| [7] | Ranjbar M., Mahmoudi Ch. & Jahaniyan S. 2016. A synopsis of the cytogenetics of the genus Phlomoides (Lamiaceae) in Iran. Caryologia, 2016. – P. 11. |
| [8] | Koparkar R. 2019. Issues and Dynamics of the Fergana Valley: Regional Implications (Monograph). New Delhi. P. 8-15. |
| [9] | Ўзбекистон Миллий Энциклопедияси, - Т.: Ўзбекистон Миллий энциклопедияси, 2005. 9-том, - Б. 200. |
| [10] | Sennikov A.N., Lazkov G.A. Taxonomic corrections and new records in vascular plants of Kyrgyzstan 2 // Memoranda Soc. Fauna Flora Fennica, 2013. – Vol. 89: – P. 132-134. |
| [11] | Lazkov G.A. 2016. Labiatae (Family Labiatae Juss.) in Flora of Kyrgyzstan (Monography). – Korea. P. 180–215. |
| [12] | Swofford D.L. 2002: PAUP*: Phylogenetic Analysis Using Parsimony (*and other methods). Version. 4.0b10. Sinauer Associates. -Sunderland. |
| [13] | Farris J. 1969. A successive approximations approach to character weighting. Systematic Biology 18: 374-385. |
| [14] | Felsenstein J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. 39: 783-791. |
| [15] | Maddison W, Maddison D. 2015. Mesquite: a modular system for evolutionary analysis. Version 3.02, http://mesquiteproject.org. |
| [16] | Махмедов А.М. Губоцветние Средней Азии (систематика, география и филогенея, ботанико-географическо и флорогенетический анализ). Докт. биол. наук. // Т.: 1991. 217 – 220 С. |






 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML