-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Virology and Molecular Biology
p-ISSN: 2163-2219 e-ISSN: 2163-2227
2021; 10(1): 1-4
doi:10.5923/j.ijvmb.20211001.01
Received: Mar. 18, 2021; Accepted: Apr. 16, 2021; Published: Apr. 30, 2021

Brain Morphological Changes in the Experimental Hypothyroidism Correction
Muminova Guyokhon1, Isroilov Rajabboy2, Inoyatova Feruza2, Abdullaeva Mashkhura2
1Andijan State Medical Institute, Andijan, Uzbekistan
2Tashkent Medical Academy, Tashkent, Uzbekistan
Correspondence to: Muminova Guyokhon, Andijan State Medical Institute, Andijan, Uzbekistan.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The developmental pathomorphological changes in the cerebral cortex and hippocampal tissue by introducing L-thyroxine and neuroprotectants into experimental hypothyroid-induced animals were studied in this research work. Treatment of hypothyroid animals with L-thyroxine for 10 days showed that perivascular and pericellular tumors remained in the cerebral cortex tissue, and colliquation foci appeared in some areas of the brain substance.
Keywords: Hypothyroidism, L-thyroxine, Neuroprotectors, Hippocampus
Cite this paper: Muminova Guyokhon, Isroilov Rajabboy, Inoyatova Feruza, Abdullaeva Mashkhura, Brain Morphological Changes in the Experimental Hypothyroidism Correction, International Journal of Virology and Molecular Biology, Vol. 10 No. 1, 2021, pp. 1-4. doi: 10.5923/j.ijvmb.20211001.01.
Article Outline
1. Introduction
- In recent years, there has been an increase in the incidence of endocrine diseases, especially thyroid disease. Among thyroid diseases, the proportion of hypothyroidism is large, accounting for 3–8% of the total population, and the majority of patients are adults, the elderly, young children, pregnant women [1,2]. Hypothyroidism - occurs with damage to all organs and organ systems of the body, including changes in the activity of the nervous system [3,4,5,6]. Among the pathological changes of the nervous system, neurological disorders in various cases of hypothyroidism also occupy a leading position.The interest of neurologists in the problem of hypothyroidism is characterized by a wide range of neurological manifestations in the early stages of the disease, associated with its impact on all levels of the nervous system [2,7]. In hypothyroidism, cognitive function decreases, intelligence, memory and concentration deteriorate, dissomnik syndrome develops, and sometimes depression is observed [8]. The main forms of neurological disorders in patients with primary hypothyroidism due to various causes are: encephalopolyneuropathy (69.8%), neuromuscular disorders (49.4%), encephalopathy (25.1%), encephalomyelopolyneuropathy (22.2%) and others include [9,10]. Epileptic seizures or fainting may occur in almost 20% of patients with hypothyroidism [10].Neurological disorders resulting from hypothyroidism are now becoming more prevalent and over time will become one of the top priorities. Therefore, improving their diagnosis and treatment methods has become one of the most pressing tasks of neurology.
2. Purpose of the Research
- Detection of morphological changes in the brain and hippocampus in thyroid hypofunction and their correction.
3. Materials and Methods
- Research material. In order to restore the morphological changes in the brain in experimental hypothyroidism, the drug "somazina" (Spanish pharmaceutical company "FERRER Internacional, S.A.") and "Neuromak" (pharmaceutical company "Radix NPP" in Uzbekistan) were selected in combination with L-thyroxine.Research design. The study used adult male rats weighing 180-220 g without 120 white females fed a standard ration in the central laboratory of TashFarMI. All animal studies were conducted in accordance with WHO recommendations for working with experimental animals and adherence to precautionary measures. To achieve the goal, the state of hypothyroidism was modeled by injecting mercazolyl into the stomach of white rats at a dose of 2.5 mg/100 g [11]. The development of hypothyroidism was confirmed by monitoring body temperature, body weight gain, and general condition of the animals, and by detecting TTG, T3, and T4 hormones. Rats with hypothyroidism were divided into 4 groups: Group 1 - control; Group 2 - treated with thyroxine; Group 3 was treated with thyroxine and a neuroprotector "neuromak". In this article, only the second and third groups were examined.Research methods: The animals were anesthetized by decapitation and fragments from the cerebral cortex and hippocampus were obtained. Brain tissue fragments were solidified for 72 h in a 10% phosphate buffer solution of formalin. It was then dewatered, poured into paraffin, bricks were prepared, and histological incisions were made. Histological incisions were stained with hematoxylin-eosin and Nissl stains, examined under a light microscope, the desired areas were photographed.
4. Results and Discussion
- In experimental hypothyroid-induced animals, L-thyroxine-neuroprotectants were administered to study the developing pathomorphological changes in the cerebral cortex and hippocampal tissue. Treatment of hypothyroid animals with L-thyroxine for 10 days showed that perivascular and pericellular tumors persisted in the cerebral cortex tissue, colliculitis foci appeared in some areas of the brain substance. It should be noted that the darkening of the nucleus and cytoplasm, which are part of neurons and glial cells, hyperchromasis (Fig. 1), especially the increase in chromatin in the nucleus, indicates an increase in protein metabolism in these cells, while the tumor process is preserved in brain tissue. The fact that the pyramidal cells from the neurons are hyperchromasia, the granular cells are stained with a relatively light color in the cytoplasm and nucleus, indicates a lack of chromatin in them (Fig. 2).
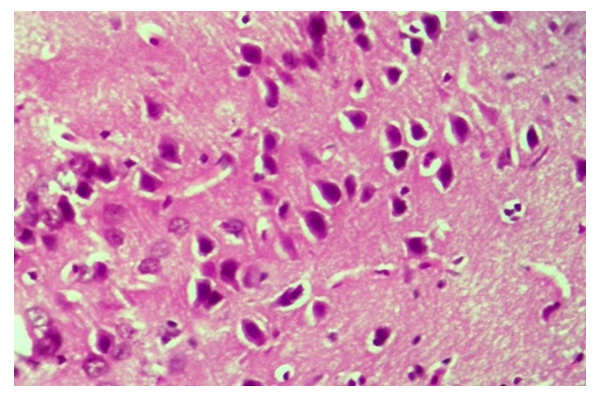 | Figure 1. Thyroxine-treated group, cerebral cortex. Preservation of perivascular and pericellular tumors, hyperchromasis of neurons. Paint: G-E. X: 10x40 |
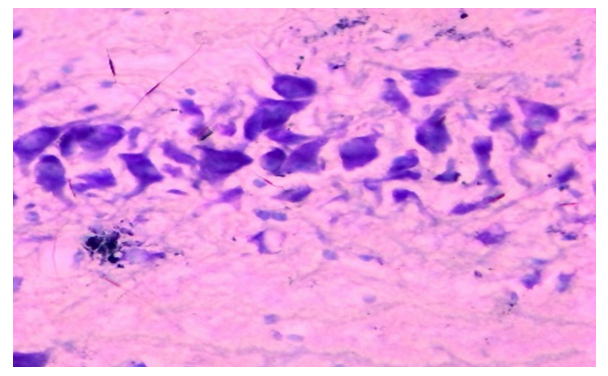 | Figure 2. Thyroxine-injected group, day 10, hippocampus. The amount of tigroid substance in the cytoplasm of neurons is increased. Dye: Nissl method. X: 10x90 |
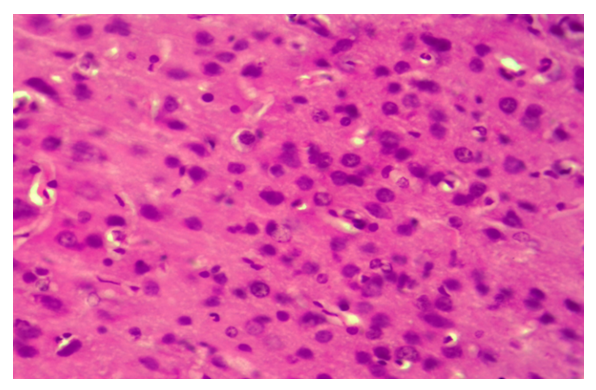 | Figure 3. Correction group with thyroxine and neuroma, day 10, cerebral cortex. There is no tumor in the brain tissue, all neurons are hypertrophic and hyperchromatic. Paint: G-E. X: 10x40 |
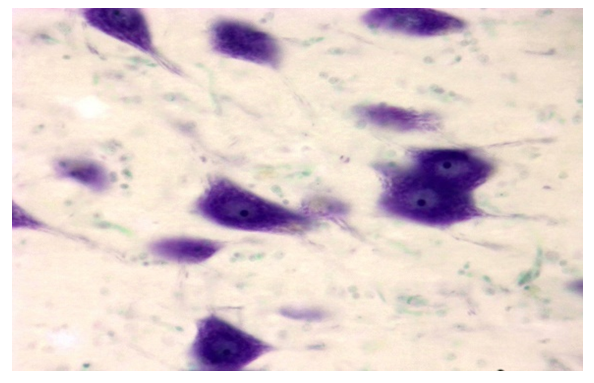 | Figure 4. Correction group with thyroxine and neuroma, day 10, cerebral cortex. The cytoplasm of neurons is filled with tigroid substance. Dye: Nissl method. X: 10x90 |
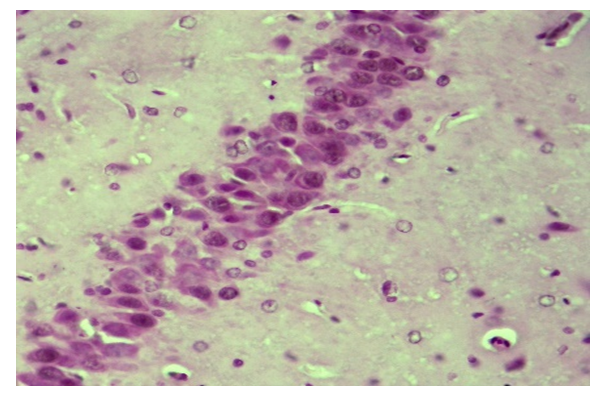 | Figure 5. Correction group with thyroxine and neuromac, 10-day, hippocampus. Hypertrophy and hyperchromasis of neurons. Paint: G-E. X: 10x40 |
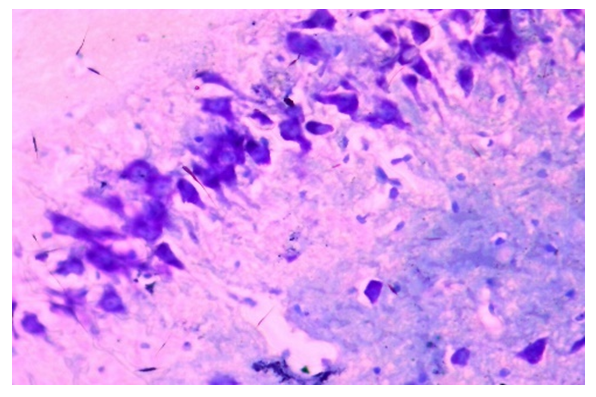 | Figure 6. Correction group with thyroxine and neuromac, 10-day, hippocampus. An increase in thyroid substance in the cytoplasm of neurons. Dye: Nissl method. X: 10x40 |
5. Conclusions
- In conclusion, the correction of experimental hypothyroidism with L-thyroxine at a dose of 3 μg/kg resulted in relatively preserved circulatory and tumor processes in the cerebral cortex and hippocampal tissue, but increased activity of enzyme and protein metabolism under the influence of L-thyroxine in neurons and glial cells, confirms that both the cytoplasm is hypertrophic and hyperchromatic. There is an increase in tigroid substance in neurons.The experimental hypothyroidism was corrected by a combination of thyroxine and neuroma, in contrast to the thyroxine-treated group, distillation and tumor processes in the brain and hippocampal tissue were stabilized and reduced from the beginning of the experiment. Absence of perivascular, pericellular tumors in the cerebral cortex and hippocampal tissue, activation of the morphofunctional state of both neurons and glial cells, hypertrophy and hyperprombosis, stimulation of neuroprotectors, cell membrane structures indicates.Thus, in thyroid hypofunction depending on the duration of the experiment, swelling of the brain and hippocampus, cell dystrophy, loss of tigroid substance were observed, reduction of L-thyroxine and neurodegenerative changes, especially with the use of neuroprotectants, activation of morphofunctional status of glial cells.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML