-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Virology and Molecular Biology
p-ISSN: 2163-2219 e-ISSN: 2163-2227
2020; 9(3): 45-49
doi:10.5923/j.ijvmb.20200903.01
Received: Nov. 8, 2020; Accepted: Nov. 27, 2020; Published: Dec. 15, 2020

Distribution of HPV Strains among HIV Positive Women in Pointe-Noire and Dolisie (Congo)
Franck Gaëtan Loubanou Tchibinda1, Luc Magloire Anicet Boumba2, 3, 4, Ghislain Loubano –Voumbi2, 5, Parfait Christy Nganga2, Arsène Lenga1, Rachel Moyen1, Donatien Moukassa2
1Faculty of Sciences and Techniques, University Marien NGOUABI, Brazzaville, Congo
2Faculty of Health Sciences, University Marien NGOUABI, Brazzaville, Congo
3Laboratory of Medical and Morphological Analysis, General Hospital of Loandjili, Pointe-Noire, Congo
4National Institute for Research in Health Sciences, Research Unit n° 9 of Dolisie, Congo
5Medical Analysis Laboratory, Dolisie General Hospital, Pointe-Noire, Congo
Correspondence to: Franck Gaëtan Loubanou Tchibinda, Faculty of Sciences and Techniques, University Marien NGOUABI, Brazzaville, Congo.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Cervical cancer is most common in women in sub-Saharan Africa. Numerous data in the literature suggest that HIV is a factor promoting the oncogenic process of HPVs. However, no study has been carried out in Congo. The objective of this study will be to assess the distribution of HPV strains among HIV positive women in Pointe-Noire and Dolisie. We carried out a descriptive and cross-sectional study over a period of 22 months between March 2017 and December 2018. After cytological analysis of the samples and serological screening of our study population, we carried out a molecular study by GeneXpert. Our results showed 5.38% of HIV-positive women in Pointe-Noire and 1.54% in Dolisie. In addition, we noted HSIL and ICC cervical lesions in HIV-positive women in Pointe-Noire (0.77% and 3.08% respectively) and 0.77% both types of lesions in Dolisie. Overall, we observed that all HIV positive women were HPV positive. Finally, HPV16 was the most determined strain in HIV women, ie 5.38% in Pointe-Noire and 1.54% in Dolisie. Our results confirm that HIV positive women are all carriers of one of the HPV strains and / or associated with high grade lesions or invasive cervical cancer. This relationship could suggest that HIV is a factor favoring the oncogenic character of HPVs.
Keywords: Distribution, HPV strains, HIV, Pointe-Noire and Dolisie
Cite this paper: Franck Gaëtan Loubanou Tchibinda, Luc Magloire Anicet Boumba, Ghislain Loubano –Voumbi, Parfait Christy Nganga, Arsène Lenga, Rachel Moyen, Donatien Moukassa, Distribution of HPV Strains among HIV Positive Women in Pointe-Noire and Dolisie (Congo), International Journal of Virology and Molecular Biology, Vol. 9 No. 3, 2020, pp. 45-49. doi: 10.5923/j.ijvmb.20200903.01.
Article Outline
1. Introduction
- Cancer of the cervix (CC) is the fourth most common cancer in women worldwide and the most common cancer in women in sub-Saharan Africa [1]. Human Papilloma virus (HPV) and Human Immunodeficiency Virus (HIV) coinfection is a major public health problem in sub-Saharan Africa [2,3]. Precancerous lesions of the cervical epithelium are the basis for the development of this cancer following oncogenic viral strains of HPV. These lesions are easily detectable by cytological screening and genotyping of the HPV strains by molecular biology. However, about 70% of infections go away in about a year, and 90% of infections go away after two years. The spontaneous regression of the infection without any lesion developing and suggests a very effective specific local immunity [4]. However, 10% of women remain infected and develop precancerous alterations of the cervical epithelium and possibly cervical cancer, making persistent HPV infection the major risk factor for cervical cancer [5,6]. In fact, types 16 and 18 are found in more than 90% of cervical cancer cases [6]. In Congo-Brazzaville, HPV 16 is the most common type found in Congolese women [7]. In addition, numerous data from the literature have noted numerous exogenous factors associated with carcinogenesis by HPV16 [7]. Additionally, although numerous studies have evaluated the relationship between the pathogenesis of HIV and HPV, the results have not been synthesized. While the antiretroviral (ART) decreases the incidence of other AIDS-related cancers, its relationship to CC is unclear [8] and particularly in sub-Saharan African countries where HIV seroprevalence remains high.Thus, a better understanding of the relationship between HIV and HPV would make it possible to better understand not only prevention and treatment, but especially the mechanisms that govern the oncogenic process of HPV. In this study, our objective will be to assess the distribution of HPV strains in HIV positive women in Pointe-Noire and Dolisie.
2. Materials and Methods
2.1. Ethical Considerations
- An ethical authorization has been obtained from the Ethics Committee for Research in Health Sciences (C.R.S.SA) and administrative authorization was provided by the establishments where the study was conducted. Written informed consent was provided by each participant, and the results were provided to participants free of charge for their clinical benefit.
2.2. Period and Study Population
- We conducted a 22-month period between March 2017 and December 2018, a descriptive and cross-sectional study in the cities of Pointe-Noire and Dolisie. All procedures were carried out at Dolisie and Loandjili General Hospital in Pointe-Noire. The selected participants were enlightened, aged 18 to 50, reported at least one episode of intercourse in the past 3 months, and were seropositive on the rapid test and HIV ELISA. The participants excluded from the study are those who are pregnant, during their period.
2.3. Sampling
- The consecutive non-probability sampling method was used in this study. A minimum sample size of 130 patients was required to achieve study results. After giving their consent, a standard questionnaire was formulated to all participants, covering socio-demographic characteristics, gyneco-obstetric and reproductive history. Whole and cervical blood samples were taken; a cervico-vaginal smear reading was taken.
2.4. HIV Testing
- HIV screening was carried out according to a National algorithm involving a sensitive rapid test (determine HIV 1/2, Alere-Japan) and an HIV ELISA test (Vironostika HIV Uni-Form II Ag / Ab, BIOMERIEUX) carried out according to the protocols of the maker.
2.5. HPV Detection and Genotyping
- All HPV tests were performed on cervical cell samples stored in SurePath liquid medium at the laboratory of Loandjili Hospital in Pointe Noire. PCR was performed using the GeneXpert-Cepheid system using the "Xpert-HPV" kit. The system allows the performance of real-time PCR flexibly. Cepheids allow the identification of high-risk oncogenic genotypes only. HPV 16, HPV 18/45 and other oncogenic HPVs are detected by this system. Extraction, amplification and detection are automated in 60 minutes.
2.6. Statistical Analyzes
- All quantifiable variables were presented as mean ± standard deviation. Excel 2010 software was used to establish our database and analyzes were performed using graph pad software version 5. The confidence interval (CI) for the statistical tests was set at 95% and the null hypothesis rejected at a threshold of 5%. Chi-square tests.
3. Results
3.1. Sociodemographic Characteristics of the Study Population
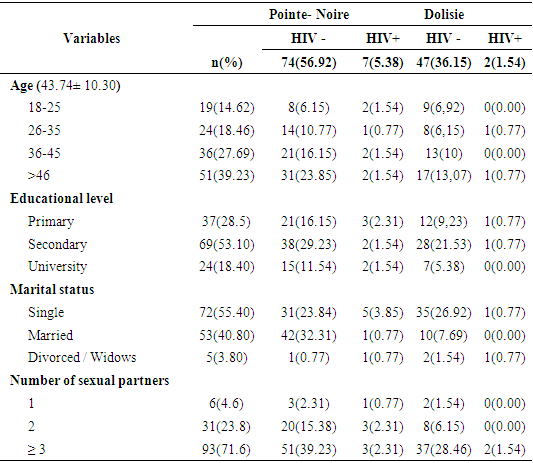
- A total of 130 women were included in this study. Analysis of our results showed a mean age of 43.74 ± 10.30 years. Overall, each age group had one positive HIV case. The level of secondary education was more representative with a prevalence of 53.10%. In addition, we noted a higher prevalence among single women, i.e. 55.40%. In addition, women with more than 2 sexual partners were more representative with 23.8%. Finally, we noted more HIV + women in Pointe-Noire than in Dolisie, ie 5.38% whose age group is between 18-25 years.
|
3.2. Cytological Results
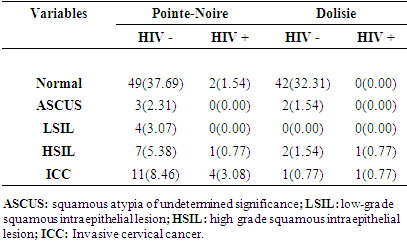
- We screened our study population for cervical lesions. Our data showed a prevalence of HSIL and ICC lesions at 0.77% and 3.08% respectively in Pointe-Noire and 0.77% of the two types of Dolisie lesions in HIV positive women.
|
3.3. Distribution of Circulating HPV Genotypes in the Study Population
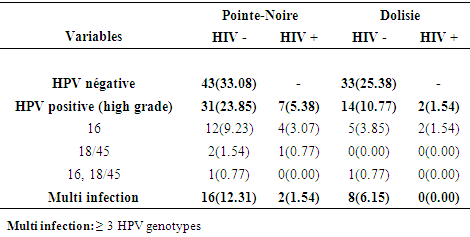
- We verified the distribution of HPV strains by Genexpert (Figure 1) according to the serological status of our study population. Our results showed that high grade HPV was globally present in all seropositive women in Pointe-Noire and Dolisie with a percentage of 5.38% and 1.54% respectively. It should also be noted that HPV16 was the majority oncogenic strain in the two serological states, i.e. 9.23% in negative women and 3.08% in positive women in Pointe-Noire and 3.85% in negative women and 1.54% among positive women in Dolisie (Table 3).
|
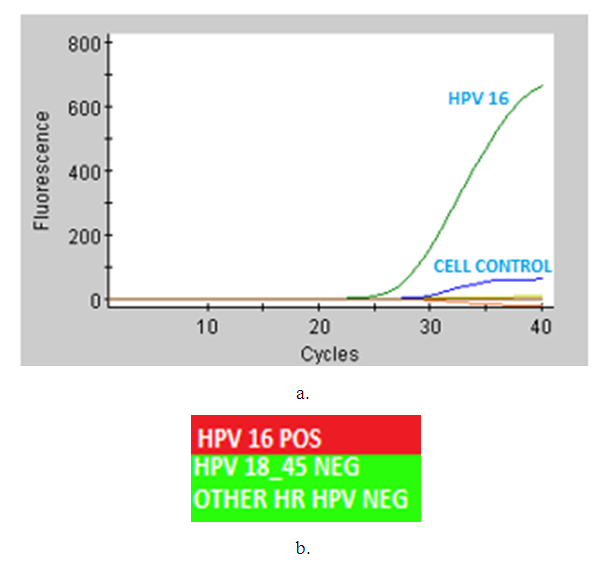 | Figure 1. a distribution of HPV strains by Genexpert |
4. Discussion
- The objective of this work was to describe the distribution of HPV strains according to HIV serological status among women in Pointe-Noire and Dolisie. We found overall HIV positivity in women of all ages. Then, we observed that women who had at least two sexual partners were more exposed to HIV infection. In addition, ICC and HSIL cervical lesions were noted in women with positive HIV status in both cities. Finally, we identified overall that all HIV positive women were additionally positive for a high grade HPV strain.This study involved 130 samples of women from the cities of Pointe-Noire and Dolisie. Although the HIV seroprevalence in these cities is respectively 9.9% in Pointe-Noire and 11.3% in Dolisie according to the National Council for the Fight against AIDS in Congo (CNLS Congo-Brazzaville), nevertheless our data have shown overall that each tranche of ages had at least one HIV positive case. Our results disagree with the study by Zacharie Ndizeye et al. in 2019 in Burundi, which had found positive HIV cases between the ages of 18 and 25. Another study from Kenya [10] showed similar results to ours for every age group that had at least one HIV positive case. These differences could be explained only by the size of the study population and the size of the city [11,12].In addition, we observed that women who had more than two sexual partners were exposed to HPV and HIV co-infection. These data are similar to several previous studies [13,14]. These results clearly show that HIV may well be a factor that may favor HPV infection by lowering immunity. Although several studies have reported an imbalance of the cervical microenvironment by inflammatory cytokines; however, at the cellular level, HIV is well known to be able to modify this environment by these specific proteins [15].Our results may support the hypothesis of several genetic polymorphisms that are involved in the development of certain cancers, in particular squamous cells, such as those of the genes encoding p53 [16], interleukin 10 [17] or even the repair enzymes of DNA [18]. Previous studies show that the p53 Suppression factor is responsible for the latency of HIV in CDT4 cells and thus promotes inflammation and local immune damage [19,20]. This change in the local microenvironment by HIV would therefore promote the oncogenic process of HPVs. This could explain why all HIV positive women tested positive for high grade HPV.Also, we observed cervical lesions in HIV positive women of the ICC type at 3.08% respectively in Pointe-Noire and 0.77% in Dolisie, followed by HSIL lesions at 0.77% in Pointe-Noire and in Dolisie. These data contrast with the work of Wilbert Mbuya, et al. [21] whose prevalence of CHF was 8% and HSIL at 4%. However, a similarity was observed overall with regard to the frequency of these lesions. Compared to women whose serology is negative for HIV in our study, we can observe that the prevalence of ICC and HSIL lesions is double of HIV positive women. HIV-negative women also presented other types of lesions including ASCUS and LSIL. These results can be explained as before by the size of the sample. Contrary to several data in the literature which incriminate immunity would be the only one responsible for the development of lesions; we can confirm in this study that HIV is not only a factor disrupting the local immune system, but would promote the oncogenic process via HPV. Finally, other endogenous factors may also be involved in the development of lesions [22].Finally, we noted in our study that the prevalence of HVP was respectively 29.23% and 5.38% in seronegative and positive women in Pointe-Noire and respectively 12.31% and 1.54% in Dolisie. In addition, our results clearly show that each age group positive for HIV was positive for a strain of HPV. This finding is not far removed from studies in East Africa [23,24,25]. These results could be explained by the mechanism for the deregulation of the cervical microenvironment by HIV and other endogenous and exogenous factors, including the number of partners [15].
5. Conclusions
- In short, we can establish in this study a relationship between HIV and HPV. Then, this study confirms that HIV positive women are all carriers of one of the HPV strains and / or associated with high grade lesions or invasive cervical cancer. Finally, we can suggest that HIV is a factor favoring the oncogenic character of HPVs. Experimental studies are needed to corroborate this relationship between HIV and HVP in cervical lesions.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML